Superalloy - Superalloy
Bu maqola uchun qo'shimcha iqtiboslar kerak tekshirish. (2018 yil mart) (Ushbu shablon xabarini qanday va qachon olib tashlashni bilib oling) |

A superalloy, yoki yuqori samarali qotishma, bu qotishma uning erish nuqtasining yuqori qismida ishlash qobiliyati bilan.[1] Superalloyning bir nechta asosiy xususiyatlari juda yaxshi mexanik quvvat, qarshilik termal sudraluvchi deformatsiya, yaxshi sirt barqarorligi va qarshilik korroziya yoki oksidlanish.
Kristall tuzilishi odatda yuzga yo'naltirilgan kub (FCC) ostenitik. Bunday qotishmalarga misollar Xastelloy, Inconel, Vaspaloy, Rene qotishmalari, Incoloy, MP98T, TMS qotishmalari va CMSX bitta kristalli qotishmalari.
Superalloyning rivojlanishi asosan kimyoviy va texnologik innovatsiyalarga bog'liq edi. Superalloydlar yuqori harorat kuchini rivojlantiradi qattiq eritmani kuchaytirish va yog'ingarchilikni kuchaytirish ikkilamchi fazadan gamma-primer va karbid kabi cho'kmalar hosil bo'ladi. Kabi elementlar tomonidan oksidlanish yoki korroziyaga chidamlilik ta'minlanadi alyuminiy va xrom. Superalloydlar ko'pincha bitta kristall sifatida quyiladi don chegaralari past haroratlarda quvvatni ta'minlashi mumkin, ular sudralish qarshiligini pasaytiradi.
Bunday qotishmalar uchun asosiy dastur aerokosmik va dengizda turbinali dvigatellar. Creep odatda gaz turbinasi pichoqlarining umrini cheklovchi omil hisoblanadi.[2]
Superalloyflar juda yuqori haroratli muhandislik texnologiyasini amalga oshirishga imkon beradigan materiallardir.[3]
Kimyoviy rivojlanish
Ushbu qotishmalar yuqori haroratli dasturlarga mo'ljallanganligi sababli (ya'ni ularning shakllarini erish nuqtasiga yaqin haroratda ushlab turish) ularning sudralmoq va oksidlanishga qarshilik birinchi darajali ahamiyatga ega. Nikel (Ni) asosidagi superalloydlar noyob applications 'cho'kindilari tufayli ushbu dasturlar uchun tanlangan material sifatida paydo bo'ldi.[1][4][sahifa kerak ] Ni asosidagi ushbu superalloyimlarning xossalari ma'lum darajada boshqa ko'plab oddiy va ekzotik elementlarning qo'shilishi, shu jumladan nafaqat metallar, Biroq shu bilan birga metalloidlar va metall bo'lmagan; xrom, temir, kobalt, molibden, volfram, tantal, alyuminiy, titanium, zirkonyum, niobiy, reniy, itriyum, vanadiy, uglerod, bor yoki gafniy ishlatiladigan qotishma qo'shimchalariga bir nechta misollar. Ushbu qo'shimchalarning har biri yuqori haroratni qo'llash xususiyatlarini optimallashtirishda ma'lum bir maqsadga xizmat qilish uchun tanlangan.
Sürünmeye qarshilik, qisman, tezlikni pasayishiga bog'liq dislokatsiya kristalli strukturadagi harakat. Zamonaviy Ni asosidagi superalloyda, γ’-Ni3(Al, Ti) bosqich dislokatsion harakatga to'siq bo'lib xizmat qiladi. Shu sababli, bu γ ’ intermetalik faza, katta hajmli fraktsiyalarda mavjud bo'lganda, bu qotishmalarning tartibini va γ matritsasi bilan yuqori muvofiqligi tufayli kuchini keskin oshiradi. Ning kimyoviy qo'shimchalari alyuminiy va titanium γ ’fazasini yaratishga yordam berish. Γ ’fazasining o'lchamini ehtiyotkorlik bilan yog'ingarchilikni kuchaytiruvchi issiqlik bilan ishlov berish orqali boshqarish mumkin. Ko'pgina superalloydlar ikki fazali issiqlik bilan ishlov berish orqali ishlab chiqariladi, bu kuboidal γ ’zarrachalarining asosiy fazasi deb nomlanadigan dispersiyasini hosil qiladi va ular orasida ikkilamchi γ’ deb nomlanadi. Ushbu qotishmalarning oksidlanish qarshiligini yaxshilash uchun Al, Cr, B va Y qo'shiladi. Al va Cr sirtni passivlashtiradigan va superalloyni keyingi oksidlanishdan saqlaydigan oksidli qatlamlarni hosil qiladi, B va Y esa ushbu oksid shkalasining substratga yopishishini yaxshilash uchun ishlatiladi.[5] Cr, Fe, Co, Mo va Re imtiyozli ravishda γ matritsaga bo'linadi, Al, Ti, Nb, Ta va V imtiyozli ravishda γ 'cho'kindilarga bo'linadi va qattiq eritma mos ravishda matritsani va cho'kmalarni kuchaytiradi. Qattiq eritmani mustahkamlashdan tashqari, agar don chegaralari mavjud bo'lsa, don chegarasini mustahkamlash uchun ma'lum elementlar tanlanadi. B va Zr don chegaralarini ajratib olishga intiladi, bu esa donning chegara energiyasini pasaytiradi va natijada don chegaralarining yaxlitligi va egiluvchanligi yaxshilanadi.[6] Donni chegaralarini mustahkamlashning yana bir shakli C va karbidning oldingi, masalan Cr, Mo, W, Nb, Ta, Ti yoki Hf qo'shilishi orqali erishiladi, bu esa don chegaralarida karbidlarning yog'ishini keltirib chiqaradi va shu bilan don chegarasi siljishini kamaytiradi.
| Element | Tarkibi oralig'i (vazn%) | Maqsad |
|---|---|---|
| Ni, Fe, Co | 50-70% | Ushbu elementlar superalloyning asosiy matritsasi γ fazasini hosil qiladi. Ni zarur, chunki u ham '' ni hosil qiladi (Ni3Al). Fe va Co ning erish nuqtalari Ni ga qaraganda yuqori va eritmaning mustahkamlanishini ta'minlaydi. Fe shuningdek, Ni yoki Co ga qaraganda ancha arzon. |
| Kr | 5-20% | Cr oksidlanish va korroziyaga chidamliligi uchun zarur; u himoya oksidi Cr hosil qiladi2O3 |
| Al | 0.5-6% | Al avvalgi asosiy. Shuningdek, u himoya oksidi Al hosil qiladi2O3, Cr ga nisbatan yuqori haroratda oksidlanish qarshiligini ta'minlaydi2O3 |
| Ti | 1-4% | Ti shakllari γ ' |
| C | 0.05-0.2% | MC va M23C6 (M Ph = dmetal) karbidlar - γ bo'lmaganda kuchayish fazasi. |
| B, Zr | 0-0.1% | Bor va zirkonyum don chegaralarini mustahkamlaydi. Bir kristalli tubin pichoqlarida bu muhim emas, chunki don chegaralari yo'q |
| Nb | 0-5% | Nb γ '' hosil qilishi mumkin, pastroq (700 ° C dan past) haroratda mustahkamlovchi faza |
| Re, V, Hf, Mo, Ta | 1-10% | Qattiq eritmani kuchaytirish uchun (va karbid hosil bo'lishi uchun) oz miqdorda qo'shiladigan o'tga chidamli metallar. Ular og'ir, ammo juda yuqori erish nuqtalariga ega |
Ni asosidagi superalloydlar yuqori haroratli juda yaxshi materiallar ekanligi va juda foydali ekanligi isbotlangan bo'lsa-da, Co asosli superalloydlar potentsial ravishda yuqori darajadagi issiq korroziyaga, oksidlanishga va aşınma qarshiligiga ega. Shu sababli, so'nggi bir necha yil ichida Co-asosidagi superalloyni ishlab chiqarishga harakat qilindi. Shunga qaramay, an'anaviy Co-asosidagi superalloydlar keng qo'llanilmadi, chunki ular Ni-ga asoslangan superalloyumalarga qaraganda yuqori haroratda past kuchga ega.[9] Buning asosiy sababi shundan iboratki, yaqin vaqtgacha ular tarkibida Ni asosidagi superalloyimlarning yuqori harorat kuchida juda muhim bo'lgan yog'ingarchilikni kuchaytirish etishmas edi. Metastable γ'-Co haqida 2006 yilgi hisobot3(Al, W) L1 bilan intermetalik birikma2 tuzilishi an'anaviy Ni asosli superalloydlarga alternativ sifatida Co asosidagi qotishmalarni taklif qiladi. Ammo bu qotishmalar klassi 1971 yilda C. S. Li tomonidan doktorlik dissertatsiyasida bayon qilingan.[10] Ikki fazali mikroyapı uzluksiz γ matritsaga kiritilgan kuboidal γ ’cho'kmalaridan iborat va shuning uchun morfologik jihatdan Ni asosidagi superalloyda kuzatilgan mikroyapı bilan bir xildir. Ni asosidagi tizim singari, ikki faza o'rtasida yuqori darajadagi izchillik mavjud bo'lib, bu yuqori haroratlarda yuqori quvvatga olib keladigan asosiy omillardan biridir. Bu og'ir muhitda qo'llash uchun yuk ko'taruvchi Co asosidagi superalloyimlarning yangi sinfini yaratish yo'lini beradi.[11] Ushbu qotishmalarda W γ ’intermetalik birikmani hosil qilish uchun hal qiluvchi qo'shimcha hisoblanadi; bu ularni ancha zichroq qiladi (> 9,6 g / sm)3) Ni asosidagi superalloydlarga nisbatan. So'nggi paytlarda k-bal 'asosidagi kobalt asosli yangi supero'tkazmalar sinfi ishlab chiqildi, ular Wsiz va zichligi ancha past, nikel asosidagi superalloyimlar bilan solishtirish mumkin.[12][13][14][15] Ushbu yangi Co-asosli superalloydlarning ko'plab xususiyatlari Ni-ga asoslangan an'anaviylarga qaraganda yaxshiroq bo'lishi mumkinligi bilan bir qatorda, Co ning erish harorati Ni ga qaraganda yuqori. Shuning uchun, agar yuqori harorat kuchini yaxshilash mumkin bo'lsa, Co asosidagi yangi superalloydlarning ishlab chiqarilishi reaktiv dvigatelning ish harorati oshishiga va natijada samaradorlikning oshishiga imkon beradi.
Faza shakllanishi
Yangi elementlarni qo'shish odatda qattiq eritmani kuchaytirish tufayli yaxshi bo'ladi, ammo muhandislar qaysi fazalar cho'kib ketishiga ehtiyot bo'lishlari kerak. Cho'kindilar geometrik jihatdan o'ralgan (GCP) deb tasniflanishi mumkin, topologik jihatdan yaqin (TCP) yoki karbidlar. GCP fazalari odatda mexanik xususiyatlar uchun yaxshi, ammo TCP fazalari ko'pincha zararli hisoblanadi. TCP fazalari chindan ham yaqin bo'lmaganligi sababli, ularda sirpanish tizimlari kam va juda mo'rt. Ular qo'shimcha ravishda yomon, chunki ular elementlarni GCP fazalaridan uzoqlashtiradilar. D 'hosil qilish uchun yaxshi bo'lgan yoki qattiq eritmani mustahkamlovchi ko'plab elementlar TCP ni cho'ktirishi mumkin. Muhandislar TCP-lardan qochishda GCP-larni targ'ib qiluvchi muvozanatni topishlari kerak.
TCP fazasi hosil bo'lgan qotishma maydoni zaif bo'ladi, chunki[16][17]
- TCP fazasi o'ziga xos sifatsiz mexanik xususiyatlarga ega
- TCP fazasi γ matritsasi bilan mos kelmaydi
- TCP fazasi dep 'bo'lmagan "tükenme zonasi" bilan o'ralgan.
- TCP fazasi odatda o'tkir plastinka yoki ignaga o'xshash morfologiyalarni hosil qiladi, ular yoriqlarni osongina nukleatsiya qiladi
Asosiy GCP fazasi γ 'dir. Ushbu faza tufayli deyarli barcha superalloydlar Ni-ga asoslangan. γ '- bu buyurtma qilingan L12 (L-one-two deb talaffuz qilinadi), ya'ni birlik hujayraning yuzida ma'lum bir atom va birlik hujayrasining burchaklarida ma'lum bir atom bor. Ni asosidagi superalloydlar uchun bu odatda yuzlarda Ni, burchaklarda Ti yoki Al degan ma'noni anglatadi.
GCP ning yana bir "yaxshi" bosqichi - bu '' '. Bundan tashqari, u with bilan izchil, ammo u yuqori haroratda eriydi.
| Bosqich | Tasnifi | Tuzilishi | Tarkibi (lar) | Tashqi ko'rinishi | Effekt |
|---|---|---|---|---|---|
| γ | matritsa | tartibsiz FCC | Ni, Co, Fe va qattiq eritmadagi boshqa elementlar | Boshqa yog'ingarchiliklar uchun fon | Matritsa fazasi cho'kmalar uchun egiluvchanlik va tuzilishni ta'minlaydi |
| γ ' | GCP | L12 (FCC buyurtma qilingan) | Ni3(Al, Ti) | kublar, yumaloq kublar, sharlar yoki trombotsitlar (panjaraning mos kelmasligiga qarab) | Asosiy mustahkamlash bosqichi. γ 'egiluvchanlikka imkon beradigan γ bilan izchil. |
| Karbid | Karbid | FCC | mC, m23C6va m6C (m Ph = -metal) | marvarid iplari singari ipga o'xshash to'plamlar | Ko'plab karbidlar mavjud, ammo ularning barchasi dispersiyani kuchaytirish va don chegaralarini barqarorlashtirishni ta'minlaydi |
| γ '' | GCP | D022 (buyurtma qilingan BCT) | Ni3Nb | juda kichik disklar | Ushbu cho'kma γ 'bilan izchil. Bu IN-718 da asosiy kuchaytirish bosqichi, ammo γ '' yuqori haroratda eriydi |
| η | GCP | D024 (buyurtma qilingan HCP) | Ni3Ti | uyali yoki Vidmanstätten naqshlarini hosil qilishi mumkin | Faza eng yomoni emas, lekin γ 'kabi yaxshi emas. Bu don chegaralarini nazorat qilishda foydalidir |
| δ | yopiq emas | ortorombik | Ni3Nb | akikulyar (ignaga o'xshash) | Ushbu bosqichning asosiy masalasi shundaki, u $ phi $ bilan izchil emas, lekin u tabiatan kuchsiz emas. Odatda u parchalanishdan hosil bo'ladi '', lekin ba'zida don chegarasini aniqlash uchun ataylab oz miqdorda qo'shiladi |
| σ | TCP | tetraedral | FeCr, FeCrMo, CrCo | cho'zilgan globuslar | Ushbu TCP odatda eng yomon mexanik xususiyatlarga ega deb hisoblanadi.[18] Mexanik xususiyatlar uchun bu hech qachon istalmagan |
| m | TCP | olti burchakli | Fe2Nb, Co2Ti, Fe2Ti | globuslar yoki trombotsitlar | Ushbu bosqich odatda TCP muammolariga ega. Mexanik xususiyatlar uchun bu hech qachon istalmagan |
| Sevadi | TCP | rombohedral | (Fe, Co)7(Mo, V)6 | qo'pol Widmanstätten trombotsitlari | Ushbu bosqich odatda TCP muammolariga ega. Mexanik xususiyatlar uchun bu hech qachon istalmagan |
Superalloy oilalari
Ni asosidagi superalloyimlarning tarixi va rivojlanishi
Qo'shma Shtatlar 1905 yilda gaz turbinasini ishlab chiqarishga qiziqish bildirgan.[1] 1910-1915 yillarda gaz turbinalaridagi yuqori harorat uchun ostenitik (fazali) zanglamaydigan po'latlar ishlab chiqarildi. 1929 yilga kelib 80Ni-20Cr qotishmasi odatiy hol bo'lib, unga Ti an Al qo'shimchalari qo'shildi. Dastlabki metallurglar buni hali bilmagan bo'lsalar-da, ular Ni asosidagi superalloyda kichik γ 'cho'kmalar hosil qilar edi. Ushbu qotishmalar tezda karbidlar va qattiq eritmani kuchaytirish bilan mustahkamlangan Fe va Co asosidagi superalloyimlardan oshib ketdi.
Cr qotishmalarni oksidlanish va korroziyadan 700 ° C gacha himoya qilish uchun juda yaxshi bo'lgan bo'lsa-da, metallurglar Cr ni oksidlanishga chidamliligi (lekin korroziyaga chidamliligi yo'q!) Bo'lgan Al foydasiga kamaytira boshladilar. Cr etishmasligi issiq korroziya bilan bog'liq muammolarni keltirib chiqardi, shuning uchun qoplamalar ishlab chiqarilishi kerak edi.
1950 atrofida, vakuum bilan eritish tijoratlashtirildi, bu esa metallurglarga yanada aniqroq tarkibli yuqori toza qotishmalar yaratishga imkon berdi.
60-70-yillarda metallurglar o'zlarining e'tiborini qotishma kimyosidan qotishmalarni qayta ishlashga o'zgartirdilar. Yo'nalish bo'yicha qotish ustunli yoki hatto bitta kristalli turbinali pichoqlarga ruxsat berish uchun ishlab chiqilgan. Oksid dispersiyasini kuchaytirish juda nozik donalar va superplastiklikka ega bo'lishi mumkin.
Ni asosidagi superalloy fazalari
- Gamma (b): Ushbu faza Ni asosidagi superalloyning matritsasini hosil qiladi. Bu qotishma elementlarning qattiq eritmasi fcc ostenitik fazasi.[18][19] Tijorat Ni asosidagi qotishmalarning ko'pchiligida joylashgan qotishma elementlari, C, Cr, Mo, W, Nb, Fe, Ti, Al, V va Ta. Ushbu materiallarning hosil bo'lishi paytida, eritmalardan Ni-qotishmalar sovutilganda, karbidlar cho'kishni boshlaydi, hatto pastroq haroratlarda γ 'fazasi cho'kadi.[19][20]
- Gamma prime (γ '): Ushbu bosqich qotishmani mustahkamlash uchun ishlatiladigan cho'kma hosil qiladi. Bu intermetalik Ni asosidagi faza3(Ti, Al) FCC L1 buyurtma qilingan2 tuzilishi.[18] Γ 'fazasi, qafas parametri 0,5% atrofida o'zgarib turadigan superalloyfrit matritsasi bilan izchil. Ni3(Ti, Al) kub yuzlarida Ni atomlari, yoki Al yoki Ti atomlari kub qirralarida joylashgan tartiblangan tizimlardir. Γ 'zarralari yig'ilib, ular kuboidal tuzilmalarni hosil qiluvchi <100> yo'nalishlar bo'yicha tekislanib, o'zlarining energiya holatlarini kamaytiradi.[19] Ushbu faza 600 ° C dan 850 ° C gacha bo'lgan beqarorlik oynasiga ega, uning ichida '' HCP η fazasiga aylanadi. 650 ° C dan past haroratlarda qo'llanilishi uchun, γ "fazasini kuchaytirish uchun ishlatish mumkin.[21]

- Gamma ikki baravar (prime "): Ushbu faza odatda Ni tarkibiga ega3Nb yoki Ni3V va g 'ga nisbatan past haroratlarda (<650 ° C) Ni asosidagi superalloyni mustahkamlash uchun ishlatiladi. G "ning kristalli tuzilishi tanaga yo'naltirilgan tetragonal (BCT), va faza 60 nm dan 10 nm gacha disklarda (001) tekisliklar bilan "in" dagi {001} oilasiga parallel ravishda. " anizotrop disklar natijasida hosil bo'ladi panjaraning mos kelmasligi o'rtasida BCT cho'kma va FCC matritsa. Bu panjaraning mos kelmasligi yuqori darajaga olib boradi izchillik shtammlari bilan birga qattiqlashishni buyurtma qilish, asosiy kuchaytirish mexanizmlarini o'z ichiga oladi. Γ "fazasi taxminan 650 ° C dan yuqori darajada beqaror.[21]
- Karbid fazalari: Karbid hosil bo'lishi odatda zararli hisoblanadi, ammo Ni asosidagi superalloyda ular yuqori haroratlarda deformatsiyaga qarshi materialning strukturasini barqarorlashtirish uchun ishlatiladi. Karbidlar don chegaralarida harakatni inhibe qiluvchi don chegaralarida hosil bo'ladi.[18][19]
- Topologik jihatdan o'ralgan (TCP) fazalar: atama "TCP bosqichi" fazalar oilasining har qanday a'zosiga taalluqlidir (shu jumladan ph fazasi, ph fazasi, m fazasi va Sevgi bosqichi ) atomik jihatdan o'ralmagan, lekin ba'zi bir samolyotlarga ega HCP yig'ish. TCP fazalari yuqori mo'rt bo'lishga moyilligi va kuchaytirish matritsasini susaytirishi bilan tavsiflanadi, qattiq eritma olovga chidamli elementlar (shu jumladan Cr, Co, W va Mo). Ushbu fazalar kinetika natijasida uzoq vaqtdan so'ng (ming soat) yuqori haroratda (> 750 ° C) hosil bo'ladi.[21]
MAR-M 247 nikel asosidagi superalloy 800 va 900 ° S haroratda juda yaxshi charchoq ko'rsatkichlariga ega edi.[22]
Co-asosidagi superalloyimlarning tarixi va rivojlanishi
Tarixiy asosda Co-asosidagi superalloydlar mexanik xususiyatlar uchun karbid yog'inlari va qattiq eritmaning mustahkamlanishiga bog'liq edi. Ushbu kuchaytirish mexanizmlari yog'ingarchilikni gamma-primer () ') kuchaytirishdan kam bo'lsa ham,[1] kobalt eritish harorati hozirda hamma joyda uchraydigan nikel asosidagi superalloyimlarga qaraganda yuqori va issiq korroziyaga chidamliligi va issiqlik charchoqlariga ega. Natijada, karbid bilan mustahkamlangan Co asosidagi superalloydlar past turg'unlikda, yuqori turg'unliklarda, masalan, gaz turbinalaridagi statsionar qanotlarda qo'llaniladi.[23]
Biroq, so'nggi tadqiqotlar shuni ko'rsatdiki, kobalt mumkin γ 'fazasini namoyish eting. Aslida, birinchi marta γ 'ning mavjudligi 1971 yil nomzodlik dissertatsiyasida sodir bo'lgan,[10] ammo hech qachon nashr etilmagan. Γ / γ 'mikroyapısı qayta kashf qilindi va birinchi marta 2006 yilda Sato va boshq.[9] Ushbu "faz" Co edi3(Al, V). Bundan tashqari, Mo, Ti, Nb, V va Ta ning γ 'fazaga, Fe, Mn va Cr esa mat matritsaga bo'linishi aniqlandi.
Co-asosidagi superalloydlarning keyingi oilasi 2015 yilda Makineni va boshq. Ushbu oila o'xshash γ / γ 'mikroyapılarına ega, ammo volframsiz va Co ning γ' fazasiga ega3(Al, Mo, Nb).[12] Volfram juda og'ir element bo'lganligi sababli, volframni yo'q qilish Co-asosidagi qotishmalarni past zichlik ayniqsa muhim bo'lgan samolyotlar uchun turbinalarda tobora hayotiy qiladi.
Yaqinda kashf etilgan superalloyimlar oilasi Nyshadham va boshqalarning yuqori mahsuldorlik tadqiqotida hisoblab chiqilgan edi.[24] 2017 yilda va Reyes Tirado va boshqalar tomonidan laboratoriyada namoyish etilgan. 2018 yilda.[15] Ushbu γ 'fazasi yana volframsiz va tarkibida Co bor3(Nb, V) va Co3(Ta, V).
Birgalikda asoslangan superalloy fazalari
- Gamma (γ): Ni asosidagi superalloydlarga o'xshash, bu superalloy matritsasining fazasi. Tijorat maqsadlarida Ni asosidagi super qotishmalar darajasida ishlatilmasa-da, tadqiqotlarda topilgan qotishma elementlari C, Cr, W, Ni, Ti, Al, Ir va Ta.[9][25] Xrom, shuningdek, gaz turbinalarida moddiy foydalanish uchun juda muhim bo'lgan oksidlanish va korroziyaga chidamliligini ta'minlaydigan Kobalt asosidagi superalloyda ishlatiladi (vaqti-vaqti bilan 20% gacha).[26]
- Gamma Prime (γ '): Xuddi Ni asosidagi super qotishmalarda bo'lgani kabi, bu faz ham qotishmani mustahkamlash uchun ishlatiladigan cho'kma hosil qiladi. Bunday holda, odatda L1 bilan o'raladi2 Co tuzilishi3Ti yoki fcc Co.3Ta, garchi ikkala W va Al bu kuboidal cho'kmalarga juda yaxshi qo'shilgan bo'lsa. Ta, Nb va Ti elementlari γ ’fazaga birlashadi va uni yuqori haroratlarda barqarorlashtirishda juda samarali. Bu barqarorlik juda muhimdir, chunki barqarorlikning yo'qligi Co-asosli superalloydlarni yuqori haroratlarda ularning Ni-asos qarindoshlaridan zaifroq bo'lishiga olib keladigan asosiy omillardan biridir.[9][27]
- Karbid fazalari: Karbid hosil bo'lishida odatdagidek, uning Co-asosidagi superalloyda paydo bo'lishi yog'ingarchilikning qattiqlashishini ta'minlaydi, ammo past haroratli egiluvchanlikni pasaytiradi.[25]
- Topologik jihatdan qadoqlangan (TCP) fazalar ba'zi bir Co-asosidagi superalloyimalarda ham paydo bo'lishi mumkin, ammo bu qotishmalarni muhandislikning muhim nuqtasi TCPlardan saqlanishdir.
Fega asoslangan superalloy fazalari
Po'lat qotishmalarda po'latlarni ishlatish qiziqish uyg'otadi, chunki ba'zi po'lat qotishmalari Ni asosidagi superaljilarga o'xshash suzuvchi va oksidlanish qarshiligini ko'rsatgan, shu bilan birga ishlab chiqarish ancha arzon.
Gamma (b): Ni asosidagi superalloydlar tarkibidagi fazalar singari Fe asosli qotishmalar ham ostenit temirning (FCC) matritsali fazasiga ega. Ushbu zanglamaydigan po'latdan yasalgan qotishmalarda uchraydigan qotishma elementlariga quyidagilar kiradi: Al, B, C, Co, Cr, Mo, Ni, Nb, Si, Ti, W va Y.[28] Al oksidlanish foydalari uchun kiritilgan bo'lsa-da, Al qo'shimchalari past og'irlikdagi fraksiyonlarda saqlanishi kerak (wt.%), Chunki Al ferritik (BCC) birlamchi faza matritsasini stabillashtiradi, bu superalloy mikroyapılarda istalmagan faza, chunki u pastroq austenitik (FCC) birlamchi fazali matritsa tomonidan namoyish etilgan yuqori harorat kuchi.[29]
Gamma-prime (γ ’): Ushbu faz qotishmani mustahkamlash uchun cho'kma sifatida kiritiladi. Ni asosidagi qotishmalar singari, Al, Ni, Nb va Ti qo'shimchalarining to'g'ri muvozanati bilan γ'-Ni3Al cho'kmalarini kiritish mumkin.
Fe asosidagi superalloyimlarning mikroyapısı
Austenitik zanglamaydigan po'latlarning ikkita asosiy turi mavjud va ular po'lat yuzasida hosil bo'lgan oksidli qatlam bilan tavsiflanadi: xrom hosil qiluvchi yoki alumina hosil qiluvchi zanglamaydigan po'lat. Xrom hosil qiluvchi zanglamaydigan po'lat eng keng tarqalgan zanglamaydigan po'latdir. Shu bilan birga, xrom hosil qiluvchi po'latlar yuqori ish haroratida, ayniqsa, suv bug'lari bo'lgan muhitda, Ni asosidagi superalloyudlarga nisbatan yuqori suzishga qarshilik ko'rsatmaydi. Yuqori ish haroratida suv bug'iga ta'sir qilish xrom hosil qiluvchi qotishmalarda ichki oksidlanishning ko'payishiga va uchuvchan Cr (oksi) gidroksidlarning tez hosil bo'lishiga olib kelishi mumkin, bu ikkalasi ham qotishmaning chidamliligi va umrini pasaytirishi mumkin.[29]
Alyuminiy oksidi hosil qiluvchi ostenitik zanglamaydigan po'latlarda po'lat yuzasida alumina oksidi bo'lgan ostenitli temirning bir fazali matritsasi (FCC) mavjud. Alumina xromiyaga qaraganda kislorodda termodinamik jihatdan ancha barqarordir. Ammo, odatda, cho'kma fazalari kuch va suzishga chidamliligini oshirish uchun kiritiladi. Alyuminiy oksidi hosil qiluvchi po'latlarda, himoya alumina qatlamini saqlab turish uchun Al suv omborlari vazifasini bajaradigan NiAl cho'kmalari kiritiladi. Bundan tashqari, Nb va Cr qo'shimchalari NiAl ning cho'kma hajmini ko'paytirish orqali alyuminiy oksidi hosil bo'lishiga va stabillashishiga yordam beradi.[29]
Aluminiy oksidi hosil qiluvchi Fe-asos superalloyimalarini rivojlantirish bo'yicha olib borilgan izlanishlar kamida 5 gradusli alyuminiy oksidi hosil qiluvchi ostenitik (AFA) qotishmalarini ko'rsatdi, ular havoda oksidlanishda har xil ish harorati + 10% suv bug'lari bilan:[30]
- AFA darajasi: (50-60) Fe- (20-25) Ni- (14-15) Cr- (2.5-3.5) Al- (1-3) Nb wt.% Asos
- Havoda oksidlanishda 750-800 ° S ish harorati + 10% suv bug'i
- Kam nikel AFA darajasi: 63Fe-12Ni-14Cr-2.5Al-0.6Nb-5Mn3Cu wt.% Bazasi
- Havodagi oksidlanishda 650 ° S ish harorati + 10% suv bug'i
- Yuqori samaradorlik AFA darajasi: (45-55) Fe- (25-30) Ni- (14-15) Cr (3.5-4.5) Al- (1-3) Nb- (0.02-0.1) Hf / Y wt% tayanch
- Havodagi oksidlanishda 850-900 ° S ish harorati + 10% suv bug'i
- AFA darajasi: (35-50) Fe- (25-35) Ni-14Cr- (3.5-4) Al-1Nb wt.% Asos
- Havoda oksidlanishda 750-1100 ° S ish harorati + 10% suv bug'i, Ni wt% ga bog'liq.
- AFA superalloy (40-50) Fe- (30-35) Ni- (14-19) Cr- (2.5-3.5) Al-3Nb
- Havoda oksidlanishda 750-850 ° S ish harorati + 10% suv bug'i
Havoda oksidlanish va suv bug'lari bo'lmagan ish haroratining yuqori bo'lishi kutilmoqda. Bundan tashqari, AFA superalloy darajasi nikel asosidagi UNS N06617 qotishmasiga yaqinlashib kelayotgan suzish kuchini namoyish etgan.
Superalloydlarning mikroyapısı
Sof Ni3Al faza atomlar alyuminiy kubik hujayraning tepasiga joylashtirilgan va sublattiyani hosil qiladi. Nikel atomlari yuzlarning markazlarida joylashgan va B pastki qatlamini hosil qiladi. stexiometrik. Subtitralardan birida ortiqcha bo'sh joylar bo'lishi mumkin, bu esa stexiometriyadan chetga chiqishga olib keladi. Γ'-fazaning A va B pastki tagliklari boshqa elementlarning katta qismini eritishi mumkin. Qotishma elementlari b fazada ham eritiladi. Γ'-faza qotishma ni noodatiy mexanizm orqali qattiqlashtiradi rentabellik anomaliyasi. Dislokatsiyalar γ'-fazada dissotsilanib, an hosil bo'lishiga olib keladi fazaga qarshi chegara. Yuqori haroratda, fazaga qarshi chegara (APB) bilan bog'liq bo'lgan erkin energiya, agar u tasodifan ruxsat etilgan siljish tekisligi bo'lmagan ma'lum bir tekislikda yotsa, sezilarli darajada kamayadi. APB past energiyali tekislikda yotishi uchun APB o'zaro faoliyat sirpanishlarini chegaralaydigan qisman dislokatsiyalar to'plami va bu past energiyali tekislik ruxsat etilgan siljish tekisligi bo'lmaganligi sababli, dissotsiatsiyalangan dislokatsiya endi samarali ravishda qulflangan. Ushbu mexanizm yordamida s'-faza Ni ning oqim kuchi3Al aslida ortadi taxminan 1000 ° C gacha bo'lgan haroratda, superalloyda ularning yuqori haroratga teng bo'lmagan kuchini beradi.
In pichoqni qo'llash uchun dastlabki material tanlovi gaz turbinasi dvigatellari kabi qotishmalarni o'z ichiga olgan Nimonik 1940 yillarda seriyali qotishmalar.[4][sahifa kerak ] Dastlabki Nimonik seriyalarga γ 'Ni kiritilgan3(Al, Ti) yog'ingarchilik matritsada, shuningdek har xil metall-uglerodda karbidlar (masalan, Cr23C6) da don chegaralari[31] qo'shimcha don chegarasi mustahkamligi uchun. Turbin pichog'ining tarkibiy qismlari edi qalbaki qadar vakuum induksiyasi kasting texnologiyalar 1950-yillarda joriy qilingan.[4][sahifa kerak ] Ushbu jarayon tozalikni sezilarli darajada yaxshilab, nuqsonlarni kamaytirdi va materialning mustahkamligi va harorat qobiliyatini oshirdi.
Zamonaviy superalloydlar 1980-yillarda ishlab chiqilgan. Birlashtirilgan birinchi avlod superalloyulari ko'paygan alyuminiy, titanium, tantal va niobiy Ushbu qotishmalardagi γ 'hajmini ko'paytirish uchun tarkib Birinchi avlod superalloyudlariga quyidagilar kiradi: PWA1480, René N4 va SRR99. Bundan tashqari, hajm ulushi γ 'cho'kmalarining bitta kristalli yoki monokristalli qotish texnikasi paydo bo'lishi bilan taxminan 50-70% gacha ko'tarildi (qarang. Bridgman texnikasi ) don chegaralarini kastingdan butunlay chiqarib tashlashga imkon beradigan superalloyflar uchun. Materialda don chegaralari bo'lmaganligi sababli, karbidlar don chegarasini mustahkamlovchi sifatida keraksiz edi va shu bilan yo'q qilindi.[4][sahifa kerak ]
Ikkinchi va uchinchi avlod superalloydlari vazni 3 va 6 foizni tashkil etdi Reniy, haroratni oshirish uchun. Re sekin diffuzor va odatda diffuziya tezligini pasaytiradigan γ matritsaga bo'linadi (va shu bilan yuqori harorat sudralmoq ) va yuqori harorat ko'rsatkichlarini yaxshilash va ikkinchi va uchinchi avlod superalloyda mos ravishda xizmat haroratini 30 ° C va 60 ° C ga oshirish.[32] Re, shuningdek, d 'fazasining (kuboidal cho'kmalardan farqli o'laroq) rafti hosil bo'lishiga yordam beradi. Sallarning borligi ularning ichida suzish tezligini pasaytirishi mumkin kuch-qonun rejimi (dislokatsiya ko'tarilishi bilan boshqariladi), lekin dominant mexanizm zarrachalarni qirqish bo'lsa, shuningdek, suzish tezligini oshirishi mumkin. Bundan tashqari, Re mo'rtlashishni shakllantirishga yordam beradi TCP bosqichlari, bu Co, W, Mo va ayniqsa Cr ni kamaytirish strategiyasiga olib keldi. Ni asosidagi superalloydlarning yosh avlodlari shu sababli Cr tarkibini sezilarli darajada kamaytirgan, ammo Cr ning kamayishi bilan oksidlanishga qarshilik. Yo'qotishni qoplash uchun zamonaviy qoplama texnikasi qo'llanilmoqda oksidlanishga qarshilik kamaygan Cr tarkibiga hamroh bo'ladi.[21][33] Ikkinchi avlod superalloyudlariga PWA1484, CMSX-4 va René N5 kiradi. Uchinchi avlod qotishmalariga CMSX-10 va René N6 kiradi. To'rtinchi, beshinchi va hattoki oltinchi avlod superalloyimalari ishlab chiqilgan ruteniy qo'shimchalar, ularni avvalgi avlod tarkibidagi Qayta o'z ichiga olgan qotishmalarga qaraganda qimmatroq qiladi. Ru ning TCP fazalarini ilgari surishdagi ta'siri yaxshi aniqlanmagan. Dastlabki hisobotlarda Ru matritsada Re ning to'yinganligini pasaytirgani va shu bilan TCP fazalarining shakllanishiga sezgirligi pasayganligi aniqlandi.[34] Yaqinda o'tkazilgan tadqiqotlar teskari ta'sirni qayd etdi. Chen va boshq., Ikkita qotishmada faqat Ru tarkibida (USTB-F3 va USTB-F6) sezilarli darajada farq qilishini aniqladiki, Ru qo'shilishi ikkala bo'linish koeffitsientini va Cr va Re ning γ matritsasida super to'yinganlikni oshirdi, va shu bilan TCP fazalarining shakllanishiga yordam berdi.[35]
Hozirgi tendentsiya juda qimmat va juda og'ir elementlardan qochishdir. Misol Eglin po'lati, harorat darajasi va kimyoviy qarshiligi buzilgan byudjet materiallari. U tarkibida reniy yoki ruteniy mavjud emas va tarkibida nikel miqdori cheklangan. Ishlab chiqarish xarajatlarini kamaytirish uchun uni kimyoviy usulda paqirda eritish uchun mo'ljallangan (garchi vakuumli krujkada yaxshilangan xususiyatlarga ega bo'lsa). Bundan tashqari, issiqlik bilan ishlov berishdan oldin an'anaviy payvandlash va quyish mumkin. Dastlabki maqsad yuqori sifatli, arzon bomba qutilarini ishlab chiqarish edi, ammo material konstruktiv dasturlarda, shu jumladan zirhlarda ham keng qo'llanilishini isbotladi.
Yagona kristalli superalloydlar
Bir kristalli superalloydlar (SX yoki SC superalloyflar) a shaklida hosil bo'ladi bitta kristall yo'naltirilgan qotish texnikasining o'zgartirilgan versiyasidan foydalangan holda, shuning uchun yo'q don chegaralari materialda. Ko'pgina boshqa qotishmalarning mexanik xususiyatlari don chegaralarining mavjudligiga bog'liq, ammo yuqori haroratda ular ishtirok etishadi sudralmoq va boshqa mexanizmlar bilan almashtirilishi kerak. Bunday qotishmalarning ko'pida buyurtma qilingan orollar intermetalik faza tartibsiz faza matritsasida o'tiradi, barchasi bir xil kristall panjaraga ega. Bu taxminan dislokatsiya -hech qanday tanishtirmasdan don chegaralarini belgilash harakati amorf qattiq tuzilishga.
Yagona kristalli (SX) superal qotishmalar aeroportlar va sanoat gaz turbinalari dvigatellarining yuqori bosimli turbinali uchastkasida xususiyatlari va ishlashning o'ziga xos kombinatsiyasi tufayli keng qo'llaniladi. Yagona kristalli quyish texnologiyasi joriy etilgandan buyon SX qotishma ishlab chiqarish haroratni oshirishga qaratilgan bo'lib, qotishma ishlashining sezilarli yaxshilanishi yangi qotishma elementlari, shu jumladan reniy (Qayta) va ruteniy (Ru).[36]
Turbinaga kirish harorati oshib borishi bilan, bunday o'ta og'ir sharoitda (ya'ni yuqori harorat va yuqori stress) yagona kristalli superalloyimlarning burish deformatsiyasi paytida yuzaga keladigan fizik hodisalar to'g'risida asosiy tushunchaga ega bo'lish muhimdir. Superalloy yagona kristalining suzib yurish deformatsiyalari yuqori harorat, stress, yo'nalish va qotishmaga bog'liq. Bir kristalli superalloyda har xil harorat va stress rejimlarida sudraluvchi deformatsiyaning 3 xil rejimi mavjud: Rafting, Uchlamchi va Birlamchi.[37][sahifa kerak ] SX qotishmalari past haroratda (~ 750 ° C) asosan birlamchi sudralib yurish xususiyatlarini namoyish etadi. Matan va boshq. dastlabki burilish deformatsiyasining darajasi valentlik o'qi va <001> / <011> simmetriya chegarasi orasidagi burchakka bog'liqdir degan xulosaga keldi.[38] 850 ° C dan yuqori haroratda uchinchi darajali sudralib yurish ustunlik qiladi va kuchlanishni yumshatish xatti-harakatiga yordam beradi.[4][sahifa kerak ] Harorat 1000 ° C dan oshganda, rafting effekti keng tarqalgan bo'lib, unda kubik zarralari tortishish stressi ostida tekis shakllarga aylanadi[39] Raflar, shuningdek, valentlik o'qiga perpendikulyar ravishda hosil bo'ladi, chunki ph fazasi vertikal kanallardan va gorizontal kanallarga ko'chirildi. <001> yo'naltirilgan CMSX-4 monokristalli superalloyning 1105 ° C va 100 MPa haroratda unaksial suzish deformatsiyasini o'tkazgandan so'ng, Reed va boshq. rafting sudralib yurish hayoti uchun foydalidir, chunki u sudraluvchi shtamm evolyutsiyasini kechiktiradi. Bundan tashqari, rafting tez sodir bo'ladi va kritik shtammga kelguncha sudraluvchi shtamm to'planishini bostiradi.[40]
Superal qotishmalardagi oksidlanish
Yuqori haroratlarda ishlaydigan va ta'sirlanadigan superalloyflar uchun korroziv atrof-muhit, oksidlanish xatti-harakati eng muhim tashvishdir. Oksidlanish qotishma elementlarning kislorod bilan yangi hosil bo'lishiga kimyoviy reaktsiyalarini o'z ichiga oladi oksid fazalar, odatda metall yuzasida. Agar oksidlanish susaytirilmagan bo'lsa, qotishma vaqt o'tishi bilan turli yo'llar bilan buzilishi mumkin, jumladan:[41][42]
- ketma-ket oksidlanish, yorilish va chayqalish vaqt o'tishi bilan qotishma eroziyasiga olib keladigan sirt.
- mo'rtlashish yoriqlar hosil bo'lishiga yordam beradigan va oksidli fazalarni kiritish orqali yuzaning charchoq muvaffaqiyatsizlik
- tükenmek super qotishma mexanik xususiyatlariga ta'sir qiluvchi va ehtimol uning ishlashiga putur etkazadigan asosiy qotishma elementlari.
Ushbu zararli jarayonlarni cheklash uchun ishlatiladigan asosiy strategiya selektiv oksidlanish deb ataladi. Sodda qilib aytganda, qotishma shunday tuzilganki, qotishma elementlarning nisbati keyinchalik oksidlanish uchun to'siq bo'lib xizmat qilishi mumkin bo'lgan maxsus oksid fazasini hosil bo'lishiga yordam beradi. Odatda, alyuminiy va xrom bu rolda ishlatiladi, chunki ular nisbatan ingichka va doimiy oksidli qatlamlarni hosil qiladi alumina (Al2O3) va xromiya (Cr2O3) navbati bilan. Bundan tashqari, ular kam kislorodga ega diffuzivliklar, ushbu qatlam ostidagi oksidlanishni samarali ravishda to'xtatadi. Ideal holda oksidlanish 2 bosqichda davom etadi. Birinchidan, vaqtincha oksidlanish turli xil elementlarning, ayniqsa ko'pchilik elementlarning (masalan, nikel yoki kobalt) konversiyasini o'z ichiga oladi. Vaqtinchalik oksidlanish qurbonlik elementining selektiv oksidlanishi to'liq to'siq qatlamini hosil qilguncha davom etadi.[41]
Selektiv oksidlanishning himoya ta'sirini ko'plab mexanizmlar buzishi mumkin. Qurbonlik oksidi qatlamining uzluksizligi tufayli mexanik buzilish tufayli buzilishi mumkin stress yoki natijasida buzilishi mumkin kinetika oksidlanish (masalan, kislorodning tarqalishi juda tez bo'lsa). Agar qatlam doimiy bo'lmasa, uning kislorod uchun diffuziya to'sig'i sifatida samaradorligi sezilarli darajada kamayadi. Oksid qatlamining barqarorligiga boshqa ozchilik elementlarning mavjudligi ham kuchli ta'sir ko'rsatadi. Masalan, ning qo'shilishi bor, kremniy va itriyum Superalloydlarga oksid qatlami yordam beradi yopishqoqlik, parchalanishni kamaytirish va himoya oksidi qatlamining yaxlitligini saqlash.[43]
Oksidlanish faqat kimyoviy degradatsiyaga uchragan superal qotishmalarning eng asosiy shakli hisoblanadi. Keyinchalik murakkab korroziya jarayonlar ish muhitida tuzlar va oltingugurt birikmalarini o'z ichiga olganida yoki kimyoviy sharoitlarda vaqt o'tishi bilan keskin o'zgarib turadigan jarayonlar keng tarqalgan. Ushbu va asosiy oksidlanish masalalari ko'pincha ingichka qoplamalar orqali hal etiladi.
Superalloyni qayta ishlash
Superalloyni qayta ishlashdagi tarixiy o'zgarishlar superalloyning sezilarli darajada o'sishiga olib keldi ish harorati. Superalloys were originally iron based and cold wrought prior to the 1940s. 1940-yillarda investitsiya kastingi of cobalt base alloys significantly raised operating temperatures. Ning rivojlanishi vacuum melting in the 1950s allowed for very fine control of the chemical composition of superalloys and reduction in contamination and in turn led to a revolution in processing techniques such as yo'naltirilgan qotish of alloys and single crystal superalloys.[44][sahifa kerak ]
There are many forms of superalloy present within a gas turbine engine, and processing methods vary widely depending on the necessary properties of each specific part.
Casting and forging
Casting and forging are traditional metallurgical processing techniques that can be used to generate both polycrystalline and monocrystalline products. Polycrystalline casts tend to have higher fracture resistance, while monocrystalline casts have higher creep resistance.
Jet turbine engines employ both poly and mono crystalline components to take advantage of their individual strengths. The disks of the high-pressure turbine, which are near the central hub of the engine are polycrystalline. The turbine blades, which extend radially into the engine housing, experience a much greater centripetal force, necessitating creep resistance. As a result, turbine blades are typically monocrystalline or polycrystalline with a preferred crystal orientation.
Investitsiyalar uchun kasting
Investitsiyalar uchun kasting is a metallurgical processing technique in which a wax form is fabricated and used as a template for a ceramic mold. Briefly, a ceramic mold is poured around the wax form, the wax form is melted out of the ceramic mold, and molten metal is poured into the void left by the wax. This leads to a metal form in the same shape as the original wax form. Investment casting leads to a polycrystalline final product, as nucleation and growth of crystal grains occurs at numerous locations throughout the solid matrix. Generally, the polycrystalline product has no preferred grain orientation.
Yo'nalish bo'yicha qotish
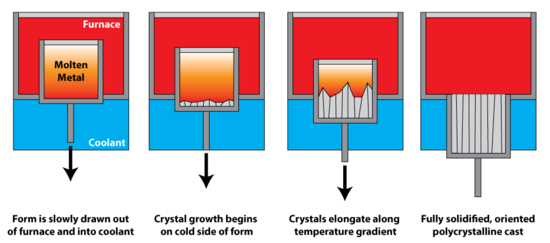
Yo'nalish bo'yicha qotish uses a thermal gradient to promote nucleation of metal grains on a low temperature surface, as well as to promote their growth along the temperature gradient. This leads to grains elongated along the temperature gradient, and significantly greater creep resistance parallel to the long grain direction. In polycrystalline turbine blades, directional solidification is used to orient the grains parallel to the centripetal force. It is also known as dendritic solidification.
Yagona kristall o'sishi
Yagona kristall o'sishi starts with a seed crystal which is used to template growth of a larger crystal. The overall process is lengthy, and additional processing via machining is necessary after the single crystal is grown.
Kukunli metallurgiya
Kukunli metallurgiya is a class of modern processing techniques in which metals are first converted into a powdered form, and then formed into the desired shape by heating below the melting point. This is in contrast to casting, which occurs with molten metal. Superalloy manufacturing often employs powder metallurgy because of its material efficiency - typically much less waste metal must be machined away from the final product—and its ability to facilitate mechanical alloying. Mexanik qotishma is a process by which reinforcing particles are incorporated into the superalloy matrix material by repeated fracture and welding.[45][tekshirib bo'lmadi ]
Sintering and hot isostatic pressing
Sinterlash va issiq izostatik presslash are processing techniques used to densify materials from a loosely packed "green body " into a solid object with physically merged grains. Sintering occurs below the melting point, and causes adjacent particles to merge at their boundaries, leading to a strong bond between them. In hot isostatic pressing, a sintered material is placed in a pressure vessel and compressed from all directions (isostatically) in an inert atmosphere to affect densification.[46]
Qo'shimchalar ishlab chiqarish
Tanlab lazer yordamida eritish (shuningdek, nomi bilan tanilgan powder bed fusion) is an additive manufacturing procedure used to create intricately detailed forms from a CAD file. In CAD, a shape is designed and then converted into slices. These slices are sent to a laser writer to print the final product. In brief, a bed of metal powder is prepared, and the first slice of the CAD design is formed in the powder bed by a high energy laser sintering the particles together. After this first slice is generated, the powder bed moves downwards, and a new batch of metal powder is rolled over the top of the slice. The second layer is then sintered with the laser, and the process is repeated until all the slices in the CAD file have been processed.[47] Due to the nature of many additive manufacturing processes, porosity can be present in products made by selective laser melting. Many products will often undergo a heat treatment or hot isostatic pressing procedure to densify the product and reduce porosity which can result in cracking.[48]
Coating of superalloys
In modern gas turbine, the turbine entry temperature (~1750K) has exceeded the incipient melting temperature of superalloys (~1600K), with the help of surface engineering. Under such extreme working condition, the qualification of coating becomes vital.[49][sahifa kerak ]
Different types of coating
Historically, three "generations" of coatings have been developed: diffusion coatings, overlay coatings and thermal barrier coatings. Diffusion coatings, mainly constituted with aluminide or platinum-aluminide, is still the most common form of surface protection. To further enhance resistance to corrosion and oxidation, MCrAlX-based overlay coatings (M=Ni or Co, X=Y, Hf, Si) are deposited to surface of superalloys. Compared to diffusion coatings, overlay coatings are less dependent on the composition of the substrate, but also more expensive, since they must be carried out by air or vacuum plasma spraying (APS/VPS)[50][sahifa kerak ] or else electron beam physical vapour deposition (EB-PVD).[51] Thermal barrier coatings provide by far the best enhancement in working temperature and coating life. It is estimated that modern TBC of thickness 300 μm, if used in conjunction with a hollow component and cooling air, has the potential to lower metal surface temperatures by a few hundred degrees.[52]
Issiqlik to'siqlarini qoplash
Thermal barrier coatings (TBCs) are used extensively on the surface of superalloy in both commercial and military gas turbine engines to increase component life and engine performance.[53] A coating of about 1-200 µm can reduce the temperature at the superalloy surface by up to 200K. TBCs are really a system of coatings consisting of a bond coat, a thermally grown oxide (TGO), and a thermally insulating ceramic top coat. In most applications, the bond coat is either a MCrAlY (where M=Ni or NiCo) or a Pt modified aluminide coating. A dense bond coat is required to provide protection of the superalloy substrate from oxidation and hot corrosion attack and to form an adherent, slow growing TGO on its surface. The TGO is formed by oxidation of the aluminum that is contained in the bond coat. The current (first generation) thermal insulation layer is composed of 7wt % ittriyada stabillashgan zirkoniya (7YSZ) with a typical thickness of 100–300 µm. Yttria stabilized zirconia is used due to its low thermal conductivity (2.6W/mK for fully dense material), relatively high coefficient of thermal expansion, and good high temperature stability. The electron beam directed vapor deposition (EB-DVD) process used to apply the TBC to turbine airfoils produces a columnar microstructure with several levels of porosity. The porosity between the columns is critical to providing strain tolerance (via a very low in-plane modulus), as it would otherwise spall on thermal cycling due to thermal expansion mismatch with the superalloy substrate. The porosity within the columns reduces the thermal conductivity of the coating.
Bond coat
The bond coat adheres the thermal barrier coating to the superalloy substrate. Additionally, the bond coat provides oxidation protection and functions as a diffusion barrier against the motion of substrate atoms towards the environment.There are five major types of bond coats, the aluminides, the platinum-aluminides, MCrAlY, cobalt-cermets, and nickel-chromium.For the aluminide bond coatings, the final composition and structure of the coating depends on the composition of the substrate. Aluminides also lack ductility below 750 °C, and exhibit a limited by thermomechanical fatigue strength. The Pt-aluminides are very similar to the aluminide bond coats except for a layer of Pt (5—10 μm) deposited on the blade. The Pt is believed to aid in oxide adhesion and contributes to hot corrosion. The cost of Pt plating is justified by the increased blade life span. The MCrAlY is the latest generation of bond coat and does not strongly interact with the substrate. Normally applied by plasma spraying, MCrAlY coatings are secondary aluminum oxide formers. This means that the coatings form an outer layer of chromium oxide (chromia), and a secondary aluminum oxide (alumina) layer underneath. These oxide formations occur at high temperatures in the range of those that superalloys usually encounter.[54] The chromia provides oxidation and hot-corrosion resistance. The alumina controls oxidation mechanisms by limiting oxide growth by self-passivating. The yttrium enhances the oxide adherence to the substrate, and limits the growth of grain boundaries (which can lead to flaking of the coating).[55] Investigation indicates that addition of rhenium and tantalum increases oxidation resistance. Kobalt -cermet based coatings consisting of materials such as volfram karbid /cobalt can be used due to excellent resistance to abrasion, corrosion, erosion, and heat.[56][to'liq iqtibos kerak ] Bular sermet coatings perform well in situations where temperature and oxidation damage are significant concerns, such as boilers. One of the unique advantages of cobalt cermet coatings is a minimal loss of coating mass over time, due to the strength of carbides within the mixture. Overall, cermet coatings are useful in situations where mechanical demands are equal to chemical demands for superalloys. Nickel-chromium coatings are used most frequently in boilers fed by Yoqilg'i moyi, elektr pechlar, and waste incineration furnaces, where the danger of oxidizing agents and corrosive compounds in the vapor must be dealt with.[57] The specific method of spray-coating depends on the composition of the coatings. Nickel-chromium coatings that also contain iron or aluminum perform much better (in terms of corrosion resistance) when they are sprayed and laser glazed, while pure nickel-chromium coatings perform better when thermally sprayed exclusively.[58]
Process methods of coating
Superalloy products that are subjected to high working temperatures and corrosive atmosphere (such as high-pressure turbine region of jet engines) are coated with various kinds of qoplama. Several kinds of coating process are applied: pack cementation process, gas phase coating (both are a type of kimyoviy bug 'cho'kmasi (CVD)), termal püskürtme va jismoniy bug 'cho'kmasi. In most cases, after the coating process near-surface regions of parts are enriched with aluminium, the matrix of the coating being nikel aluminidi.
Pack cementation process
Pack cementation is a widely used chemical vapor deposition technique which consists of immersing the components to be coated in a metal powder mixture and ammonium halide activators and sealing them in a retort. The entire apparatus is placed inside a furnace and heated in a protective atmosphere to a lower than normal temperature for diffusion to take place, due to the halide salts chemical reaction which causes an eutectic bond between the two metals. The new surface alloy that is formed due to thermal diffused ion migration has a metallurgical bond to the surface substrate and a true intermetallic layer found in the gamma layer of the new surface alloys.
The traditional pack consists of four components:
Substrate or parts- Ferrous and non-ferrousPowdered alloy- (Ti and/or Al, Si and/or Zn, B and/ or Cr)Halide salt activator- Ammonium halide saltsRelatively inert filler powder (Al2O3, SiO2, or SiC)Temperatures below (750 °C)This process includes but is not limited to:
AluminizingChromizingSiliconizingSherardizingBoronizingTitaniumizing
Pack Cementation has had a revival in the last 10 years as it is being combined with other chemical processes to lower the temperatures of metal combinations even further and give intermetallic properties to different alloy combinations for surface treatments.
Termal püskürtme
Thermal spraying is a process of applying coatings by heating a feedstock of precursor material and spraying it on a surface. Different specific techniques are used depending on desired particle size, coat thickness, spray speed, desired area, etc.[59][to'liq iqtibos kerak ] The coatings applied by thermal spraying of any kind, however, rely on adhesion to the surface. As a result, the surface of the superalloy must be cleaned and prepared, usually polished, before application of the thermal coating.[60]
Plazma bilan purkash
Of the various thermal spray methods, one of the more ideal and commonly used techniques for coating superalloys is plasma spraying. This is due to the versatility of usable coatings, and the high-temperature performance of plasma-sprayed coatings.[61] Plasma spraying can accommodate a very wide range of materials, much more so than other techniques. As long as the difference between melting and decomposition temperatures is greater than 300 Kelvin, a material can be melted and applied as a coating via plasma spraying.[62][sahifa kerak ]
Gas phase coating
This process is carried out at higher temperatures, about 1080 °C. The coating material is usually loaded onto special trays without physical contact with the parts to be coated. The coating mixture contains active coating material and activator, but usually does not contain thermal ballast. As in the pack cementation process, the gaseous aluminium chloride (or fluoride) is transferred to the surface of the part. However, in this case the diffusion is outwards. This kind of coating also requires diffusion heat treatment.
Failure mechanisms in thermal barrier coating systems
Failure of thermal barrier coating usually manifests as delamination, which arises from the temperature gradient during thermal cycling between ambient temperature and working conditions coupled with the difference in thermal expansion coefficient of the substrate and coating. It is rare for the coating to fail completely – some pieces of it remain intact, and significant scatter is observed in the time to failure if testing is repeated under identical conditions.[4][sahifa kerak ] There are various degradation mechanisms for thermal barrier coating,[63][64] and some or all of these must operate before failure finally occurs:
- Oxidation at the interface of thermal barrier coating and underlying bond coat;[65]
- The depletion of aluminum in bond coat due to oxidation[66] and diffusion with substrate;[67]
- Thermal stresses from mismatch in thermal expansion coefficient and growth stress due to the formation of thermally grown oxide layer;[68]
- Imperfections near thermally grown oxide layer;[69][70][71]
- Various other complicating factors during engine operation.[72][73][74][75][76]
Additionally, TBC life is very dependent upon the combination of materials (substrate, bond coat, ceramic) and processes (EB-PVD, plasma spraying) used.
Ilovalar
Nickel-based superalloys are used in load-bearing structures to the highest homologous temperature of any common alloy system (Tm = 0.9, or 90% of their melting point). Among the most demanding applications for a structural material are those in the hot sections of turbine engines. The preeminence of superalloys is reflected in the fact that they currently comprise over 50% of the weight of advanced aircraft engines. The widespread use of superalloys in turbine engines coupled with the fact that the thermodynamic efficiency of turbine engines is increased with increasing turbine inlet temperatures has, in part, provided the motivation for increasing the maximum-use temperature of superalloys. In fact, during the past 30 years turbine airfoil temperature capability has increased on average by about 4 °F (2.2 °C) per year. Two major factors which have made this increase possible are
- Advanced processing techniques, which improved alloy cleanliness (thus improving reliability) and/or enabled the production of tailored microstructures such as directionally solidified or single-crystal material.
- Alloy development resulting in higher-use-temperature materials primarily through the additions of refractory elements such as Re, W, Ta, and Mo.
About 60% of the use-temperature increases have occurred due to advanced cooling concepts; 40% have resulted from material improvements. State-of-the-art turbine blade surface temperatures are near 2,100 °F (1,150 °C); the most severe combinations of stress and temperature corresponds to an average bulk metal temperature approaching 1,830 °F (1,000 °C).
Although Nickel-based superalloys retain significant strength to temperatures near 1,800 °F (980 °C), they tend to be susceptible to environmental attack because of the presence of reactive alloying elements (which provide their high-temperature strength). Surface attack includes oxidation, hot corrosion, and thermal fatigue. In the most demanding applications, such as turbine blade and vanes, superalloys are often coated to improve environmental resistance.[18]
In general, high temperature materials are needed for energy conversion and energy production applications. Maximum energy conversion efficiency is desired in these energy applications, which can be achieved by increasing operating temperatures, as described by the Carnot cycle. Because Carnot efficiency is limited by the temperature difference between the hot and cold reservoirs, higher operating temperatures result in higher energy conversion efficiencies. Operating temperatures are limited by the performance of today’s superalloys, and currently, most applications operate at around 1000 °C-1400 °C. Energy applications and their superalloy components include:[77]
- Gas turbines (turbine blades)
- Solar thermal power plants (stainless steel rods containing heated water)
- Steam turbines (turbine blades and boiler housing)
- Heat exchangers for nuclear reactor systems
Alumina-forming stainless steels can be processed via melting and ladle casting, similar to the production of more common steels. Compared to vacuum casting processes, ladle casting is much less expensive. In addition, alumina-forming stainless steel has been shown to be weldable and has potential for use in high performance automotive applications, such as for high temperature exhaust piping and in heat capture and reuse.
Research and development of new superalloys
The availability of superalloys during past decades has led to a steady increase in turbine entry temperatures, and the trend is expected to continue. Sandia milliy laboratoriyalari is studying a new method for making superalloys, known as radioliz. It introduces an entirely new area of research into creating alloys and superalloys through nanoparta sintez. This process holds promise as a universal method of nanoparta shakllanish. By developing an understanding of the basic moddiy fan behind these nanoparticle formations, there is speculation that it might be possible to expand research into other aspects of superalloys.
There may be considerable disadvantages in making alloys by this method. About half of the use of superalloys is in applications where the service temperature is close to the melting temperature of the alloy. It is common therefore to use single crystals. The above method produces polycrystalline alloys, which suffer from an unacceptable level of creep.
Future paradigms in alloy development are expected to focus on weight reduction and improving oxidation and corrosion resistance while maintaining the strength of the alloy. Furthermore, with the increasing demand for turbine blades for power generation, another focus of alloy design is to reduce the cost of superalloys.
There has been ongoing research and development of new stainless steel alloys because of the lower costs in producing such alloys, as well as the need for an austenitic stainless steel with high-temperature corrosion resistance in environments with water vapor. Research is focusing on increasing high-temperature tensile strength, toughness, and creep resistance to compete with Ni-based superalloys.[30]
A new class of alumina-forming austenitic stainless steel is actively being developed for use in high-temperature applications by Oak Ridge National Laboratory. Initial research showed similar creep and corrosion resistance at 800 °C to that of other austenitic alloys, including Ni-based superalloys.[30]
Development of AFA superalloys with a 35 wt.% Ni-base have shown potential for use in operating temperatures upwards to 1,100 °C.[30]
Shuningdek qarang
Adabiyotlar
- ^ a b v d e Sims, C.T. (1984). "A History of Superalloy Metallurgy for Superalloy Metallurgists". Superalloys 1984 (Fifth International Symposium). pp. 399–419. doi:10.7449/1984/Superalloys_1984_399_419.
- ^ Carter, Tim J (April 2005). "Common failures in gas turbine blades". Muhandislik xatolarini tahlil qilish. 12 (2): 237–247. doi:10.1016/j.engfailanal.2004.07.004.
- ^ Sims, C.T. (1984). "A History of Superalloy Metallurgy for Superalloy Metallurgists". Superalloys 1984 (Fifth International Symposium). pp. 399–419. doi:10.7449/1984/Superalloys_1984_399_419.
- ^ a b v d e f Reed, R. C (2008). The Superalloys: Fundamentals and Applications. Kembrij: Kembrij universiteti matbuoti. ISBN 9780521070119.
- ^ Klein, L.; Shen, Y .; Killian, M. S.; Virtanen, S. (2011). "Effect of B and Cr on the high temperature oxidation behaviour of novel γ/γ′-strengthened Co-base superalloys". Korroziyaga qarshi fan. 53 (9): 2713–720. doi:10.1016/j.corsci.2011.04.020.
- ^ Shinagawa, K.; Omori, Toshihiro; Oikawa, Katsunari; Kainuma, Ryosuke; Ishida, Kiyohito (2009). "Ductility Enhancement by Boron Addition in Co–Al–W High-temperature Alloys". Scripta Materialia. 61 (6): 612–15. doi:10.1016/j.scriptamat.2009.05.037.
- ^ Giamei, Anthony (September 2013). "Development of Single Crystal Superalloys: A Brief History". Murakkab materiallar va jarayonlar: 26–30 – via asminternational.
- ^ Akca, Enes; Gursel, Ali (2015). "A Review on Superalloys and IN718 Nickel-Based INCONEL Superalloy". Periodicals of Engineering and Natural Sciences. 3 (1): 15–27 – via pen.ius.edu.ba.
- ^ a b v d Sato, J (2006). "Cobalt-Base High-Temperature Alloys". Ilm-fan. 312 (5770): 90–91. Bibcode:2006Sci...312...90S. doi:10.1126/science.1121738. PMID 16601187. S2CID 23877638.
- ^ a b Lee, C. S. (1971). Precipitation-hardening characteristics of ternary cobalt - aluminum - X alloys (Doktorlik dissertatsiyasi). Arizona universiteti.
- ^ Suzuki, A.; DeNolf, Garret C.; Pollock, Tresa M. (2007). "Flow Stress Anomalies in γ/γ′ Two-phase Co–Al–W-base Alloys". Scripta Materialia. 56 (5): 385–88. doi:10.1016/j.scriptamat.2006.10.039.
- ^ a b Makineni, S. K.; Nithin, B.; Chattopadhyay, K. (March 2015). "A new tungsten-free γ–γ' Co–Al–Mo–Nb-based superalloy". Scripta Materialia. 98: 36–39. doi:10.1016/j.scriptamat.2014.11.009.
- ^ Makineni, S. K.; Nithin, B.; Chattopadhyay, K. (February 2015). "Synthesis of a new tungsten-free γ–γ′ cobalt-based superalloy by tuning alloying additions". Acta Materialia. 85: 85–94. doi:10.1016/j.actamat.2014.11.016.
- ^ Makineni, S. K.; Samanta, A.; Rojhirunsakool, T.; Alam, T.; Nithin, B.; Singh, A.K .; Banerji, R.; Chattopadhyay, K. (September 2015). "A new class of high strength high temperature Cobalt based γ–γ′ Co–Mo–Al alloys stabilized with Ta addition". Acta Materialia. 97: 29–40. doi:10.1016/j.actamat.2015.06.034.
- ^ a b Reyes Tirado, Fernando L.; Perrin Toinin, Jacques; Dunand, David C. (June 2018). "γ+γ′ microstructures in the Co-Ta-V and Co-Nb-V ternary systems". Acta Materialia. 151: 137–148. doi:10.1016/j.actamat.2018.03.057.
- ^ a b Belan, Juraj (2016). "GCP and TCP Phases Presented in Nickel-base Superalloys". Bugungi materiallar: Ish yuritish. 3 (4): 936–941. doi:10.1016/j.matpr.2016.03.024.
- ^ a b Rae, C.M.F.; Karunaratne, M.S.A.; Small, C.J.; Broomfield, R.W.; Jones, C.N.; Reed, R.C. (2000). "Topologically Close Packed Phases in an Experimental Rhenium-Containing Single Crystal Superalloy". Superalloys 2000 (Ninth International Symposium). 767–776-betlar. doi:10.7449/2000/Superalloys_2000_767_776. ISBN 0-87339-477-1.
- ^ a b v d e Randy Bowman. "Superalloys: A Primer and History". Olingan 6 mart 2020 – via tms.org.
- ^ a b v d Sabol, G. P.; Stickler, R. (1969). "Microstructure of Nickel-Based Superalloys". Fizika holati Solidi (B). 35 (1): 11–52. Bibcode:1969PSSBR..35...11S. doi:10.1002/pssb.19690350102.
- ^ Doi, M.; Miki, D .; Moritani, T.; Kozakai, T. (2004). "Gamma/Gamma-Prime Microstructure Formed by Phased Separation of Gamma-Prime Precipitates in a Ni-Al-Ti Alloy". Superalloys 2004 (Tenth International Symposium). 109–114 betlar. doi:10.7449/2004/Superalloys_2004_109_114. ISBN 0-87339-576-X.
- ^ a b v d Dunand, David C. "Materials Science & Engineering 435: High Temperature Materials". Shimoliy-G'arbiy Universitet, Evanston. 25 February 2016. Lecture.
- ^ Šmíd, Miroslav; Kunz, Ludvík; Hutař, Pavel; Hrbáček, Karel (1 January 2014). "High Cycle Fatigue of Nickel-based Superalloy MAR-M 247 at High Temperatures". Processia Engineering. 74: 329–332. doi:10.1016/j.proeng.2014.06.273.
- ^ Institute, Cobalt (14 February 2018). "Superalloys". www.cobaltinstitute.org. Olingan 10 dekabr 2019.
- ^ Nyshadham, Chandramouli; Oses, Corey; Hansen, Jacob E.; Takeuchi, Ichiro; Curtarolo, Stefano; Hart, Gus L.W. (2017 yil yanvar). "A computational high-throughput search for new ternary superalloys". Acta Materialia. 122: 438–447. doi:10.1016/j.actamat.2016.09.017. S2CID 11222811.
- ^ a b Cui, C (2006). "A New Co-Base Superalloy Strengthened by γ' Phase". Materiallar bilan operatsiyalar. 47 (8): 2099–2102. doi:10.2320/matertrans.47.2099.
- ^ Coutsouradis, D.; Davin, A.; Lamberigts, M. (April 1987). "Cobalt-based superalloys for applications in gas turbines". Materialshunoslik va muhandislik. 88: 11–19. doi:10.1016/0025-5416(87)90061-9.
- ^ Suzuki, A.; Pollock, Tresa M. (2008). "High-temperature strength and deformation of γ/γ′ two-phase Co–Al–W-base alloys". Acta Materialia. 56 (6): 1288–97. doi:10.1016/j.actamat.2007.11.014.
- ^ "Review: precipitation in austenitic stainless steels". www.phase-trans.msm.cam.ac.uk. Olingan 2 mart 2018.
- ^ a b v Brady, M. P.; Yamamoto, Y .; Santella, M. L.; Maziasz, P. J.; Pint, B. A.; Liu, C. T.; Lu, Z. P.; Bei, H. (July 2008). "The development of alumina-forming austenitic stainless steels for high-temperature structural use". JOM. 60 (7): 12–18. Bibcode:2008JOM....60g..12B. doi:10.1007/s11837-008-0083-2. S2CID 137354503.
- ^ a b v d Muralidharan, G.; Yamamoto, Y .; Brady, M. P.; Walker, L. R.; Meyer III, H. M.; Leonard, D. N. (November 2016). "Development of Cast Alumina-Forming Austenitic Stainless Steels". JOM. 68 (11): 2803–2810. Bibcode:2016JOM....68k2803M. doi:10.1007/s11837-016-2094-8. OSTI 1362187. S2CID 137160315.
- ^ Bombač, D.; Fazarinc, M.; Kugler, G.; Spajić, S. (2008). "Microstructure development of Nimonic 80A superalloys during hot deformation". Materials and Geoenvironment. 55 (3): 319–328. Olingan 8 mart 2020 - ResearchGate orqali.
- ^ Reed, R. C (2006). The Superalloys: Fundamentals and Applications. Kembrij: Kembrij universiteti matbuoti. p. 121 2. ISBN 9780521070119.
- ^ Dunand, David C. "High-Temperature Materials for Energy Conversion" Materialshunoslik va muhandislik 381: Materials for Energy-Efficient Technology. Shimoliy-G'arbiy Universitet, Evanston. 3 February 2015. Lecture.
- ^ O'Hara, K. S., Walston, W. S., Ross, E. W., Darolia, R. US Patent 5482789, 1996.
- ^ Chen, J. Y .; Feng, Q .; Sun, Z. Q. (October 2010). "Topologically close-packed phase promotion in a Ru-containing single crystal superalloy". Scripta Materialia. 63 (8): 795–798. doi:10.1016/j.scriptamat.2010.06.019.
- ^ Wahl, Jacqueline; Harris, Ken (2014). "New single crystal superalloys – overview and update". MATEC konferentsiyalari. 14: 17002. doi:10.1051/matecconf/20141417002.
- ^ Nabarro, F. R. N.; de Villiers, H. L. (1995). The Physics of creep : creep and creep-resistant alloys. London: Talylor and Francis. ISBN 9780850668520.
- ^ Matan, N.; Koks, D. C .; Karter, P .; Rist, M. A.; Rae, C. M. F.; Reed, R. C. (1999). "Creep of CMSX-4 superalloy single crystals: effects of misorientation and temperature". Acta Materialia. 47 (5): 1549–1563. doi:10.1016/s1359-6454(99)00029-4.
- ^ Nabarro, Frank R. N. (1996). "Rafting in Superalloys". Metallurgiya va materiallar bilan operatsiyalar A. 27 (3): 513–530. Bibcode:1996MMTA...27..513N. doi:10.1007/BF02648942. S2CID 137172614.
- ^ Reed, R. C.; Matan, N.; Koks, D. C .; Rist, M. A.; Rae, C. M. F. (1999). "Creep of CMSX-4 superalloy single crystals: effects of rafting at high temperature". Acta Materialia. 47 (12): 3367–3381. doi:10.1016/S1359-6454(99)00217-7.
- ^ a b Pettit, F.S.; Meier, G.H. (1984). "Oxidation and Hot Corrosion of Superalloys". Superalloys 1984 (Fifth International Symposium). pp. 651–687. doi:10.7449/1984/Superalloys_1984_651_687.
- ^ Lund and Wagner. "Oxidation of Nickel- and Cobalt-Base Superalloys". DMIC report 214. 1 March 1965. Defense Metals Information Center, Batelle Memorial Institute, Columbus, Ohio.
- ^ Klein, L.; Bauer, S .; Neumeier, S.; Göken, M.; Virtanan, S. (2011). "High temperature oxidation of γ/γ'-strengthened Co-based superalloys". Korroziyaga qarshi fan. 53 (5): 2027–2034. doi:10.1016/j.corsci.2011.02.033.
- ^ C. Sims, N. Stoloff, W. Hagel, Superalloys II: High Temperature Materials for Aerospace and Industrial Power, 1987, John Wiley & Sons
- ^ "PIM International Vol. 7 No. 1 March 2013". Powder Injection Moulding International. Olingan 1 mart 2016.
- ^ Atkinson, H. V.; Davies, S. (December 2000). "Fundamental aspects of hot isostatic pressing: An overview". Metallurgiya va materiallar bilan operatsiyalar A. 31 (12): 2981–3000. Bibcode:2000MMTA...31.2981A. doi:10.1007/s11661-000-0078-2. S2CID 137660703.
- ^ Gu, D D; Meiners, W; Wissenbach, K; Poprawe, R (May 2012). "Laser additive manufacturing of metallic components: materials, processes and mechanisms". Xalqaro materiallar sharhlari. 57 (3): 133–164. doi:10.1179/1743280411Y.0000000014. S2CID 137144519.
- ^ Graybill, Benjamin; Li, Ming; Malawey, David; Ma, Chao; Alvarado-Orozco, Juan-Manuel; Martinez-Franco, Enrique (18 June 2018). "Additive Manufacturing of Nickel-Based Superalloys". Volume 1: Additive Manufacturing; Bio and Sustainable Manufacturing. College Station, Texas, USA: American Society of Mechanical Engineers. doi:10.1115/MSEC2018-6666. ISBN 978-0-7918-5135-7.
- ^ Y. Tamarin, Protective Coatings for Turbine Blades (Materials Park, OH: ASM International, 2002).
- ^ J. R. Davis, ed., Handbook of Thermal Spray Technology (Materials Park, OH: The ASM Thermal Spray Society, 2004).
- ^ Boone, D. H. (1986). "Physical vapour deposition processes". Materialshunoslik va texnologiya. 2 (3): 220–224. doi:10.1179/mst.1986.2.3.220.
- ^ Klark, Devid R. (2003 yil yanvar). "Past issiqlik o'tkazuvchanlik termal to'siq qoplamalari uchun materiallarni tanlash bo'yicha ko'rsatmalar". Yuzaki va qoplama texnologiyasi. 163-164: 67–74. doi:10.1016 / S0257-8972 (02) 00593-5.
- ^ "Wadley Research Group '". Virjiniya universiteti. Olingan 3 mart 2016.
- ^ Warnes, Bruce Michael (January 2003). "Improved aluminide/MCrAlX coating systems for super alloys using CVD low activity aluminizing". Yuzaki va qoplama texnologiyasi. 163-164: 106–111. doi:10.1016/S0257-8972(02)00602-3.
- ^ Tawancy, H.M.; Abbas, N.M.; Bennett, A. (December 1994). "Role of Y during high temperature oxidation of an M-Cr-Al-Y coating on an Ni-base superalloy". Yuzaki va qoplama texnologiyasi. 68-69: 10–16. doi:10.1016/0257-8972(94)90130-9.
- ^ D. Chuanxian; H. Bingtang; L. Huiling (24 August 1984). "Plasma-sprayed wear-resistant ceramic and cermet coating materials". Yupqa qattiq filmlar. 118 (4): 485–493. Bibcode:1984TSF...118..485C. doi:10.1016/0040-6090(84)90277-3.
- ^ Kawahara, Yuuzou (January 1997). "Development and application of high-temperature corrosion-resistant materials and coatings for advanced waste-to-energy plants". Materials at High Temperatures. 14 (3): 261–268. doi:10.1080/09603409.1997.11689552.
- ^ Longa, Y.; Takemoto, M. (July 1992). "High-Temperature Corrosion of Laser-Glazed Alloys in Na 2 SO 4 -V 2 O 5". Korroziya. 48 (7): 599–607. doi:10.5006/1.3315978.
- ^ G. R. Heath, P. Heimgartner, G. Irons, R. Miller, S. Gustafsson, Materialshunoslik forumi 1997, 251–54, 809
- ^ Knotek, O. (2001). "Thermal Spraying and Detonation Gun Processes" (PDF). In Bunshah, R. F. (ed.). Handbook of Hard Coatings: Deposition Technologies, Properties and Applications. Park Ridge, NJ: Noyes Pub.; Norwich, NY: William Andrew Pub. 77-107 betlar. ISBN 9780815514381.
- ^ Niranatlumpong, P.; Ponton, C. B.; Evans, H. E. (2000). "The Failure of Protective Oxides on Plasma-Sprayed NiCrAlY Overlay Coatings". Metalllarning oksidlanishi. 53 (3–4): 241–258. doi:10.1023/A:1004549219013. S2CID 136826569.
- ^ P. Fauchais, A. Vardelle, M. Vardelle, Modelling of Plasma Spraying of Ceramic Films and Coatings, Ed. Vinenzini, Pub. Elsevier State Publishers B.V 1991.
- ^ Evans, A. G.; Mumm, D. R.; Hutchinson, J. W.; Meier, G. H.; Pettit, F. S. (2001). "Mechanisms controlling the durability of thermal barrier coatings". Materialshunoslik sohasida taraqqiyot. 46 (5): 505–553. doi:10.1016/s0079-6425(00)00020-7.
- ^ Wright, P. K.; Evans, A. G. (1999). "Mechanisms governing the performance of thermal barrier coatings". Qattiq jismlar va materialshunoslik bo'yicha hozirgi fikr. 4 (3): 255–265. Bibcode:1999COSSM...4..255W. doi:10.1016/s1359-0286(99)00024-8.
- ^ Wright, P. K. (1998). "Influence of cyclic strain on life of a PVD TBC". Materialshunoslik va muhandislik. A245 (2): 191–200. doi:10.1016/S0921-5093(97)00850-2.
- ^ Pint, B.A. (2004 yil noyabr). "The role of chemical composition on the oxidation performance of aluminide coatings". Yuzaki va qoplama texnologiyasi. 188-189: 71–78. doi:10.1016/j.surfcoat.2004.08.007.
- ^ Baufeld, B.; Bartsch, M.; Broz, P.; Schmucker, M. (2004). "Microstructural changes as postmortem temperature indicator in Ni-Co-Cr-Al-Y oxidation protection coatings". Materialshunoslik va muhandislik. 384 (1–2): 162–171. doi:10.1016/j.msea.2004.05.052.
- ^ Nychka, J.A; Clarke, D.R (September 2001). "Damage quantification in TBCs by photo-stimulated luminescence spectroscopy". Yuzaki va qoplama texnologiyasi. 146-147: 110–116. doi:10.1016/S0257-8972(01)01455-4.
- ^ Mumm, D. R.; Evans, A. G.; Spitsberg, I. T. (2001). "Characterisation of a cyclic displacement instability for a thermally grown oxide in a thermal barrier coating system". Acta Materialia. 49 (12): 2329–2340. doi:10.1016/s1359-6454(01)00071-4.
- ^ Mumm, D. R.; Evans, A. G. (2000). "On the role of imperfections in the failure of a thermal barrier coating made by electron beam deposition". Acta Materialia. 48 (8): 1815–1827. doi:10.1016/s1359-6454(99)00473-5.
- ^ Gell, M.; Vaidyanathan, K.; Barber, B.; Cheng, J .; Jordan, E. (1999)."Platinum aluminid / elektron nurlarining fizik bug 'bilan yotqizilgan termal to'siq qoplamalarida parchalanish mexanizmi". Metallurgiya va materiallar bilan operatsiyalar A. 30 (2): 427–435. Bibcode:1999 yil MMTA ... 30..427G. doi:10.1007 / s11661-999-0332-1. S2CID 137312835.
- ^ Evans, A.G .; U, M.Y .; Xatchinson, JV (2001 yil yanvar). "Termik to'siq qoplamalarining chidamliligi uchun mexanikaga asoslangan masshtablash qonunlari". Materialshunoslik sohasida taraqqiyot. 46 (3–4): 249–271. doi:10.1016 / S0079-6425 (00) 00007-4.
- ^ Shults, U; Menzebax, M; Leyens, C; Yang, Y.Q (sentyabr 2001). "Substrat materialining oksidlanish xatti-harakatlariga va EB-PVD TBC tizimlarining tsiklik umr ko'rishiga ta'siri". Yuzaki va qoplama texnologiyasi. 146-147: 117–123. doi:10.1016 / S0257-8972 (01) 01481-5.
- ^ Chen, X; Vang, R; Yao, N; Evans, A.G; Xatchinson, JV; Bryus, RW (2003 yil iyul). "Termal to'siq tizimidagi begona narsalarning shikastlanishi: mexanizmlar va simulyatsiyalar". Materialshunoslik va muhandislik: A. 352 (1–2): 221–231. doi:10.1016 / S0921-5093 (02) 00905-X.
- ^ Uolston, V.S. (2004). "Turbinali havo plyonkalarini qoplash va sirt texnologiyalari". Superalloys 2004 (o'ninchi xalqaro simpozium). 579-588 betlar. doi:10.7449 / 2004 / Superalloys_2004_579_588. ISBN 0-87339-576-X.
- ^ Mumm, D. R .; Vatanabe, M.; Evans, A. G.; Pfaendtner, J. A. (2004). "Sinov usulining ishdan chiqish mexanizmlariga va termik to'siq tizimining chidamliligiga ta'siri". Acta Materialia. 52 (5): 1123–1131. CiteSeerX 10.1.1.514.3611. doi:10.1016 / j.actamat.2003.10.045.
- ^ Brady, M. P .; Muralidxaran, G.; Leonard, D. N .; Xeyns, J. A .; Weldon, R. G.; Angliya, R. D. (2014 yil dekabr). "Nomzodning quyma temir va zanglamaydigan po'latdan yasalgan egzoz tizimi qotishmalarini havoda 650 dan 800 ° C gacha bo'lgan suv bug'lari bilan uzoq muddatli oksidlanishi". Metalllarning oksidlanishi. 82 (5–6): 359–381. doi:10.1007 / s11085-014-9496-1. OSTI 1185421. S2CID 136677636.
Bibliografiya
- Levitin, Valim (2006). Metall va qotishmalarning yuqori harorat zo'riqishi: jismoniy asoslar. WILEY-VCH. ISBN 978-3-527-31338-9.
- Shahsavari, H. A .; Kokabi, A. H .; Nategh, S. (2007). "Oldindan yasalgan mikroyapının Rene 80 superalloyning HAZ suyuqlanish krakingiga ta'siri". Materialshunoslik va texnologiya. 23 (5): 547–555. doi:10.1179 / 174328407x179539. S2CID 135755442.
Tashqi havolalar
- "Superalloys". Kembrij universiteti. To'liq bibliografiya va havolalar.
