Stilbestrol - Stilbestrol
 | |
| Ismlar | |
|---|---|
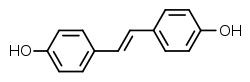
| IUPAC nomi 4-[(E) -2- (4-Gidroksifenil) etenil] fenol | |
| Boshqa ismlar Dihidroksistilben; 4,4'-Dihidroksistilben, 4,4'-stilbenediol | |
| Identifikatorlar | |
3D model (JSmol ) | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox boshqaruv paneli (EPA) | |
| |
| |
| Xususiyatlari | |
| C14H12O2 | |
| Molyar massa | 212.24388 g / mol |
| -130·10−6 sm3/ mol | |
Boshqacha ko'rsatilmagan hollar bundan mustasno, ulardagi materiallar uchun ma'lumotlar berilgan standart holat (25 ° C [77 ° F], 100 kPa da). | |
| Infobox ma'lumotnomalari | |
Stilbestrol, yoki stilboestrol, shuningdek, nomi bilan tanilgan 4,4'-dihidroksistilben yoki 4,4'-stilbenediol, a stilbenoid steroid bo'lmagan estrogen[1] va ota birikma ko'proq guruhning kuchli steroid bo'lmagan estrogen hosilalar shu jumladan, eng muhimi, dietilstilbestrol (DES).[1][2][3] "Stilbestrol" atamasi ko'pincha DESga nisbatan noto'g'ri ishlatiladi, ammo ular bir xil birikma emas.[2]
Stilbestrolning o'zi faol estrogen hisoblanadi, ammo DES va boshqa hosilalarga qaraganda kuchliroq emas.[1]
Stilbestrol hosilalari
Stilbestrol estrogenik dorilariga quyidagilar kiradi:
- Acefluranol (antiestrogen)
- Benzestrol (texnik jihatdan cho'zilgan markaziy zanjiri tufayli stilbestrol hosilasi emas, balki juda yaqin analog va har qanday holatda stilbestrol estrogenlari bilan guruhlangan)
- Bifluranol
- Dienestrol
- Dietilstilbestrol (odatda, ammo xato bilan qisqartirilib, shunchaki "stilbestrol")
- Dietilstilbestrol diatsetat
- Dietilstilbestrol dilaurat
- Dietilstilbestrol dipalmitat
- Dietilstilbestrol dipropionat
- Dietilstilbestrol disulfat
- Dietilstilbestrol monobenzil efiri
- Dimestrol (dianisilheksen, dietilstilbestrol dimetil efir, dimetoksidietilstilben)
- Fosfestrol (dietilstilbestrol difosfat)
- Furostilbestrol (dietilstilbestrol difuroat)
- ICI-85966 (dietilstilbestrol bis [di (2-xloretil) karbamat)
- Mestilbol (dietilstilbestrol monometil efiri)
- Dimetilstilbestrol
- Hexestrol (dihidrodietilstilbestrol)
- Diaetifen (hexestrol bis (2-dietilaminoetil) efir) (koronar vazodilatator)
- Geksestrol diatsetat
- Geksestrol dikaprilat
- Geksestrol difosfat
- Geksestrol dipropionat
- Fenestrol (hexestrol bis [4- [bis (2-xloroetil) amino] fenilasetat)
- Metestrol (prometestrol; dimetilheksestrol)
- Metestrol dipropionat (prometestrol dipropionat)
- Pentafluranol
- Terfluranol
Stilbestrol estrogenlari orasida dietilstilbestrol, geksestrol va benzestrol eng taniqli hisoblanadi.[4]
Ta'sir mexanizmi
Stilbestrol estrogenlari yuqori darajada bog'lanadi qarindoshlik ikkalasiga ham ERa va ERβ.[5]
| Ligand | Boshqa ismlar | Nisbatan majburiy yaqinlik (RBA,%)a | Mutlaq majburiy yaqinliklar (Kmen, nM)a | Amal | ||
|---|---|---|---|---|---|---|
| ERa | ERβ | ERa | ERβ | |||
| Estradiol | E2; 17β-Estradiol | 100 | 100 | 0.115 (0.04–0.24) | 0.15 (0.10–2.08) | Estrogen |
| Estrone | E1; 17-ketoestradiol | 16.39 (0.7–60) | 6.5 (1.36–52) | 0.445 (0.3–1.01) | 1.75 (0.35–9.24) | Estrogen |
| Estriol | E3; 16a-OH-17b-E2 | 12.65 (4.03–56) | 26 (14.0–44.6) | 0.45 (0.35–1.4) | 0.7 (0.63–0.7) | Estrogen |
| Estetrol | E4; 15a, 16a-Di-OH-17β-E2 | 4.0 | 3.0 | 4.9 | 19 | Estrogen |
| Alfatradiol | 17a-Estradiol | 20.5 (7–80.1) | 8.195 (2–42) | 0.2–0.52 | 0.43–1.2 | Metabolit |
| 16-epiyestriol | 16β-gidroksi-17β-estradiol | 7.795 (4.94–63) | 50 | ? | ? | Metabolit |
| 17-epiyestriol | 16a-gidroksi-17a-estradiol | 55.45 (29–103) | 79–80 | ? | ? | Metabolit |
| 16,17-Epiestriol | 16β-gidroksi-17a-estradiol | 1.0 | 13 | ? | ? | Metabolit |
| 2-gidroksietradiol | 2-OH-E2 | 22 (7–81) | 11–35 | 2.5 | 1.3 | Metabolit |
| 2-metoksietradiol | 2-MeO-E2 | 0.0027–2.0 | 1.0 | ? | ? | Metabolit |
| 4-gidroksietradiol | 4-OH-E2 | 13 (8–70) | 7–56 | 1.0 | 1.9 | Metabolit |
| 4-metoksyestradiol | 4-MeO-E2 | 2.0 | 1.0 | ? | ? | Metabolit |
| 2-gidroksistron | 2-OH-E1 | 2.0–4.0 | 0.2–0.4 | ? | ? | Metabolit |
| 2-metoksietron | 2-MeO-E1 | <0.001–<1 | <1 | ? | ? | Metabolit |
| 4-gidroksistron | 4-OH-E1 | 1.0–2.0 | 1.0 | ? | ? | Metabolit |
| 4-metoksietron | 4-MeO-E1 | <1 | <1 | ? | ? | Metabolit |
| 16a-gidroksietron | 16a-OH-E1; 17-ketoestriol | 2.0–6.5 | 35 | ? | ? | Metabolit |
| 2-gidroksistriol | 2-OH-E3 | 2.0 | 1.0 | ? | ? | Metabolit |
| 4-metoksistriol | 4-MeO-E3 | 1.0 | 1.0 | ? | ? | Metabolit |
| Estradiol sulfat | E2S; Estradiol 3-sulfat | <1 | <1 | ? | ? | Metabolit |
| Estradiol disulfat | Estradiol 3,17β-disulfat | 0.0004 | ? | ? | ? | Metabolit |
| Estradiol 3-glyukuronid | E2-3G | 0.0079 | ? | ? | ? | Metabolit |
| Estradiol 17β-glyukuronid | E2-17G | 0.0015 | ? | ? | ? | Metabolit |
| Estradiol 3-glyuk. 17β-sulfat | E2-3G-17S | 0.0001 | ? | ? | ? | Metabolit |
| Estrone sulfat | E1S; Estrone 3-sulfat | <1 | <1 | >10 | >10 | Metabolit |
| Estradiol benzoat | EB; Estradiol 3-benzoat | 10 | ? | ? | ? | Estrogen |
| Estradiol 17β-benzoat | E2-17B | 11.3 | 32.6 | ? | ? | Estrogen |
| Estrone metil efiri | Estrone 3-metil efir | 0.145 | ? | ? | ? | Estrogen |
| ent-Estradiol | 1-estradiol | 1.31–12.34 | 9.44–80.07 | ? | ? | Estrogen |
| Ekvilin | 7-degidroestron | 13 (4.0–28.9) | 13.0–49 | 0.79 | 0.36 | Estrogen |
| Ekvilenin | 6,8-Didehidroestron | 2.0–15 | 7.0–20 | 0.64 | 0.62 | Estrogen |
| 17β-Dihidroekvilin | 7-Dehidro-17β-estradiol | 7.9–113 | 7.9–108 | 0.09 | 0.17 | Estrogen |
| 17a-Dihidroekvilin | 7-Dehidro-17a-estradiol | 18.6 (18–41) | 14–32 | 0.24 | 0.57 | Estrogen |
| 17β-Dihidroekvilenin | 6,8-Didehidro-17b-estradiol | 35–68 | 90–100 | 0.15 | 0.20 | Estrogen |
| 17a-Dihidroekvilenin | 6,8-Didehidro-17a-estradiol | 20 | 49 | 0.50 | 0.37 | Estrogen |
| Δ8-Estradiol | 8,9-Dehidro-17β-estradiol | 68 | 72 | 0.15 | 0.25 | Estrogen |
| Δ8-Estron | 8,9-degidroestron | 19 | 32 | 0.52 | 0.57 | Estrogen |
| Etinilestradiol | EE; 17a-etinil-17β-E2 | 120.9 (68.8–480) | 44.4 (2.0–144) | 0.02–0.05 | 0.29–0.81 | Estrogen |
| Mestranol | EE 3-metil efir | ? | 2.5 | ? | ? | Estrogen |
| Moksestrol | RU-2858; 11β-Metoksi-EE | 35–43 | 5–20 | 0.5 | 2.6 | Estrogen |
| Metilestradiol | 17a-Metil-17b-estradiol | 70 | 44 | ? | ? | Estrogen |
| Dietilstilbestrol | DES; Stilbestrol | 129.5 (89.1–468) | 219.63 (61.2–295) | 0.04 | 0.05 | Estrogen |
| Hexestrol | Dihidrodietilstilbestrol | 153.6 (31–302) | 60–234 | 0.06 | 0.06 | Estrogen |
| Dienestrol | Dehidrostilbestrol | 37 (20.4–223) | 56–404 | 0.05 | 0.03 | Estrogen |
| Benzestrol (B2) | – | 114 | ? | ? | ? | Estrogen |
| Xlorotrianizen | TACE | 1.74 | ? | 15.30 | ? | Estrogen |
| Trifeniletilen | TPE | 0.074 | ? | ? | ? | Estrogen |
| Trifenilbrometilen | TPBE | 2.69 | ? | ? | ? | Estrogen |
| Tamoksifen | ICI-46,474 | 3 (0.1–47) | 3.33 (0.28–6) | 3.4–9.69 | 2.5 | SERM |
| Afimoksifen | 4-gidroksitamoksifen; 4-OHT | 100.1 (1.7–257) | 10 (0.98–339) | 2.3 (0.1–3.61) | 0.04–4.8 | SERM |
| Toremifen | 4-xlorotamoksifen; 4-CT | ? | ? | 7.14–20.3 | 15.4 | SERM |
| Klomifen | MRL-41 | 25 (19.2–37.2) | 12 | 0.9 | 1.2 | SERM |
| Siklofenil | F-6066; Seksovid | 151–152 | 243 | ? | ? | SERM |
| Nafoksidin | U-11,000A | 30.9–44 | 16 | 0.3 | 0.8 | SERM |
| Raloksifen | – | 41.2 (7.8–69) | 5.34 (0.54–16) | 0.188–0.52 | 20.2 | SERM |
| Arzoksifen | LY-353,381 | ? | ? | 0.179 | ? | SERM |
| Lasofoksifen | CP-336,156 | 10.2–166 | 19.0 | 0.229 | ? | SERM |
| Ormeloksifen | Centchroman | ? | ? | 0.313 | ? | SERM |
| Levormeloksifen | 6720-CDRI; NNC-460,020 | 1.55 | 1.88 | ? | ? | SERM |
| Ospemifen | Deaminogidroksitorememen | 2.63 | 1.22 | ? | ? | SERM |
| Bazedoksifen | – | ? | ? | 0.053 | ? | SERM |
| Etakstil | GW-5638 | 4.30 | 11.5 | ? | ? | SERM |
| ICI-164,384 | – | 63.5 (3.70–97.7) | 166 | 0.2 | 0.08 | Antiestrogen |
| Fulvestrant | ICI-182,780 | 43.5 (9.4–325) | 21.65 (2.05–40.5) | 0.42 | 1.3 | Antiestrogen |
| Propilpirazoletriol | PPT | 49 (10.0–89.1) | 0.12 | 0.40 | 92.8 | ERa agonisti |
| 16a-LE2 | 16a-lakton-17b-estradiol | 14.6–57 | 0.089 | 0.27 | 131 | ERa agonisti |
| 16a-Iodo-E2 | 16a-Iodo-17b-estradiol | 30.2 | 2.30 | ? | ? | ERa agonisti |
| Metilpiperidinopirazol | MPP | 11 | 0.05 | ? | ? | ERa antagonisti |
| Diarilpropionitril | DPN | 0.12–0.25 | 6.6–18 | 32.4 | 1.7 | ERβ agonisti |
| 8β-VE2 | 8β-Vinil-17β-estradiol | 0.35 | 22.0–83 | 12.9 | 0.50 | ERβ agonisti |
| Prinaberel | ERB-041; Yo'l-202,041 | 0.27 | 67–72 | ? | ? | ERβ agonisti |
| ERB-196 | YO'L-202,196 | ? | 180 | ? | ? | ERβ agonisti |
| Erteberel | SERBA-1; LY-500,307 | ? | ? | 2.68 | 0.19 | ERβ agonisti |
| SERBA-2 | – | ? | ? | 14.5 | 1.54 | ERβ agonisti |
| Coumestrol | – | 9.225 (0.0117–94) | 64.125 (0.41–185) | 0.14–80.0 | 0.07–27.0 | Xenoestrogen |
| Genistein | – | 0.445 (0.0012–16) | 33.42 (0.86–87) | 2.6–126 | 0.3–12.8 | Xenoestrogen |
| Teng | – | 0.2–0.287 | 0.85 (0.10–2.85) | ? | ? | Xenoestrogen |
| Daidzein | – | 0.07 (0.0018–9.3) | 0.7865 (0.04–17.1) | 2.0 | 85.3 | Xenoestrogen |
| Biochanin A | – | 0.04 (0.022–0.15) | 0.6225 (0.010–1.2) | 174 | 8.9 | Xenoestrogen |
| Kaempferol | – | 0.07 (0.029–0.10) | 2.2 (0.002–3.00) | ? | ? | Xenoestrogen |
| Naringenin | – | 0.0054 (<0.001–0.01) | 0.15 (0.11–0.33) | ? | ? | Xenoestrogen |
| 8-Prenilnaringenin | 8-PN | 4.4 | ? | ? | ? | Xenoestrogen |
| Quercetin | – | <0.001–0.01 | 0.002–0.040 | ? | ? | Xenoestrogen |
| Ipriflavon | – | <0.01 | <0.01 | ? | ? | Xenoestrogen |
| Miroestrol | – | 0.39 | ? | ? | ? | Xenoestrogen |
| Dezoksimiroestrol | – | 2.0 | ? | ? | ? | Xenoestrogen |
| b-sitosterol | – | <0.001–0.0875 | <0.001–0.016 | ? | ? | Xenoestrogen |
| Resveratrol | – | <0.001–0.0032 | ? | ? | ? | Xenoestrogen |
| a-Zearalenol | – | 48 (13–52.5) | ? | ? | ? | Xenoestrogen |
| b-Zearalenol | – | 0.6 (0.032–13) | ? | ? | ? | Xenoestrogen |
| Zeranol | a-Zearalanol | 48–111 | ? | ? | ? | Xenoestrogen |
| Taleranol | b-Zearalanol | 16 (13–17.8) | 14 | 0.8 | 0.9 | Xenoestrogen |
| Zearalenone | ZEN | 7.68 (2.04–28) | 9.45 (2.43–31.5) | ? | ? | Xenoestrogen |
| Zearalanone | ZAN | 0.51 | ? | ? | ? | Xenoestrogen |
| Bisfenol A | BPA | 0.0315 (0.008–1.0) | 0.135 (0.002–4.23) | 195 | 35 | Xenoestrogen |
| Endosulfan | EDS | <0.001–<0.01 | <0.01 | ? | ? | Xenoestrogen |
| Kepone | Chlordecone | 0.0069–0.2 | ? | ? | ? | Xenoestrogen |
| o, p '-DDT | – | 0.0073–0.4 | ? | ? | ? | Xenoestrogen |
| p, p '-DDT | – | 0.03 | ? | ? | ? | Xenoestrogen |
| Metoksiklor | p, p '-Dimetoksi-DDT | 0.01 (<0.001–0.02) | 0.01–0.13 | ? | ? | Xenoestrogen |
| HPTE | Gidroksixlor; p, p '-OH-DDT | 1.2–1.7 | ? | ? | ? | Xenoestrogen |
| Testosteron | T; 4-Androstenolon | <0.0001–<0.01 | <0.002–0.040 | >5000 | >5000 | Androgen |
| Dihidrotestosteron | DHT; 5a-Androstanolon | 0.01 (<0.001–0.05) | 0.0059–0.17 | 221–>5000 | 73–1688 | Androgen |
| Nandrolone | 19-Nortestosteron; 19-NT | 0.01 | 0.23 | 765 | 53 | Androgen |
| Dehidroepiandrosteron | DHEA; Prasterone | 0.038 (<0.001–0.04) | 0.019–0.07 | 245–1053 | 163–515 | Androgen |
| 5-Androstenediol | A5; Androstenediol | 6 | 17 | 3.6 | 0.9 | Androgen |
| 4-Androstenediol | – | 0.5 | 0.6 | 23 | 19 | Androgen |
| 4-Androstenedion | A4; Androstenedion | <0.01 | <0.01 | >10000 | >10000 | Androgen |
| 3a-Androstandiol | 3a-Adiol | 0.07 | 0.3 | 260 | 48 | Androgen |
| 3β-Androstandiol | 3β-Adiol | 3 | 7 | 6 | 2 | Androgen |
| Androstanedione | 5a-Androstedion | <0.01 | <0.01 | >10000 | >10000 | Androgen |
| Etioxolanedion | 5β-Androstedion | <0.01 | <0.01 | >10000 | >10000 | Androgen |
| Metiltestosteron | 17a-metiltestosteron | <0.0001 | ? | ? | ? | Androgen |
| Etinil-3a-androstandiol | 17a-etinil-3a-adiol | 4.0 | <0.07 | ? | ? | Estrogen |
| Etinil-3β-androstandiol | 17a-etinil-3b-adiol | 50 | 5.6 | ? | ? | Estrogen |
| Progesteron | P4; 4-Pregnenedion | <0.001–0.6 | <0.001–0.010 | ? | ? | Progestogen |
| Noretisteron | NET; 17a-etinil-19-NT | 0.085 (0.0015–<0.1) | 0.1 (0.01–0.3) | 152 | 1084 | Progestogen |
| Norethynodrel | 5 (10) -Noretisteron | 0.5 (0.3–0.7) | <0.1–0.22 | 14 | 53 | Progestogen |
| Tibolone | 7a-metilnoretinodrel | 0.5 (0.45–2.0) | 0.2–0.076 | ? | ? | Progestogen |
| Δ4-Tibolon | 7a-Metilnoretisteron | 0.069–<0.1 | 0.027–<0.1 | ? | ? | Progestogen |
| 3a-gidroksitibolon | – | 2.5 (1.06–5.0) | 0.6–0.8 | ? | ? | Progestogen |
| 3β-gidroksitibolon | – | 1.6 (0.75–1.9) | 0.070–0.1 | ? | ? | Progestogen |
| Izohlar: a = (1) Majburiy yaqinlik mavjud qiymatlarga qarab qiymatlar "median (range)" (# (# - #)), "range" (# - #) yoki "value" (#) formatida. Ushbu diapazondagi to'liq qiymatlar to'plamini Wiki kodida topish mumkin. (2) Majburiy yaqinliklar turli xil joylarni almashtirish ishlari orqali aniqlandi in-vitro bilan tizimlar belgilangan estradiol va inson ERa va ERβ oqsillar (Kuiper va boshq. (1997) dan ERβ qiymatlari bundan mustasno, ular ER rat kalamushidir). Manbalar: Shablon sahifasiga qarang. | ||||||
Stilbestrollar bilan chambarchas bog'liq bo'lgan estrogenlarni o'z ichiga oladi paroksipropion (dietilstilbestrol metaboliti) va anis va arpabodiyon - hosil bo'lgan birikmalar anol, dianol, anetol, dianetol va fotoanethole (aslida stilbestrol estrogenlari aslida kelib chiqqan). The trifeniletilen trifeniletilenni o'z ichiga olgan estrogen dorilar guruhi, estrobin, xlorotrianizen, broparestrol, etamoksitrifetol, klomifen, tamoksifen, va yaqinda ishlab chiqarilgan lotinlar ham stilbestrollar bilan tizimli ravishda chambarchas bog'liqdir.
Resveratrol texnik jihatdan silbestrol hosilasi bo'lmagan estrogen xususiyatiga ega stilbenoid (u 3,4 ', 5-stilbenetriol).[6]
Kasbiy ta'sir
Stilboestrolning kasbiy ta'siriga olib keldi ginekomastiya ishchilarda.[7]
Shuningdek qarang
Adabiyotlar
- ^ a b v Noller KL, Fish CR (1974 yil iyul). "Dietilstilbestroldan foydalanish: uning qiziqarli o'tmishi, muhim hozirgi va shubhali kelajagi". Med. Klinika. Shimoliy Am. 58 (4): 793–810. doi:10.1016 / s0025-7125 (16) 32122-8. PMID 4276416.
- ^ a b VITAMINLAR VA GORMONLAR. Akademik matbuot. 1945 yil 1-yanvar.233 –. ISBN 978-0-08-086600-0.
- ^ Uilyam Jon Edvard Jessop (2014 yil 12-may). Fearonning biokimyoga kirish. Elsevier. 408– betlar. ISBN 978-1-4831-9556-8.
- ^ Giyohvand moddalarning harakatlari va ulardan foydalanish. Stenford universiteti matbuoti. 234– betlar. ISBN 978-0-8047-1505-8.
- ^ Kuiper, Jorj G. J. M.; Karlsson, Bo; Grandien, Kaj; Enmark, Eva; Xaggblad, Yoxan; Nilsson, Stefan; Gustafsson, Jan-Ek (1997). "Ligandning bog'lash xususiyatini taqqoslash va estrogen retseptorlari a va b ning transkripsiyaviy to'qimalarining tarqalishi". Endokrinologiya. 138 (3): 863–870. doi:10.1210 / endo.138.3.4979. ISSN 0013-7227. PMID 9048584.
- ^ Bhat KP, Lantvit D, Kristov K, Mehta RG, Moon RC, Pezzuto JM; Lantvit; Xristov; Mehta; Oy; Pezzuto (2001 yil oktyabr). "Sut bezlari o'smalari modellarida resveratrolning estrogen va antiestrogen ta'sirlari". Saraton kasalligi. 61 (20): 7456–63. PMID 11606380.CS1 maint: bir nechta ism: mualliflar ro'yxati (havola)
- ^ Fitzsimons, P.M. (1944 yil oktyabr). "Ginekomastiya Stilboestrol ishchilarida". Br J Ind Med. 1 (4): 235–237. PMC 1035620.

